The Lab Guide: When to Use Soda Lime vs. Borosilicate

Glassware can be the weakest link in a perfect workflow. The right glass lets you heat samples safely, keep things clean, maintain consistent results, and reduce breakage during daily use. The wrong glass can crack during sterilization, release unwanted ions into sensitive samples, or lose strength after repeated washing and handling. Two common types you'll see in labs are soda lime glass and borosilicate glass, and they are made for very different lab needs.
Quick, Clear Definitions
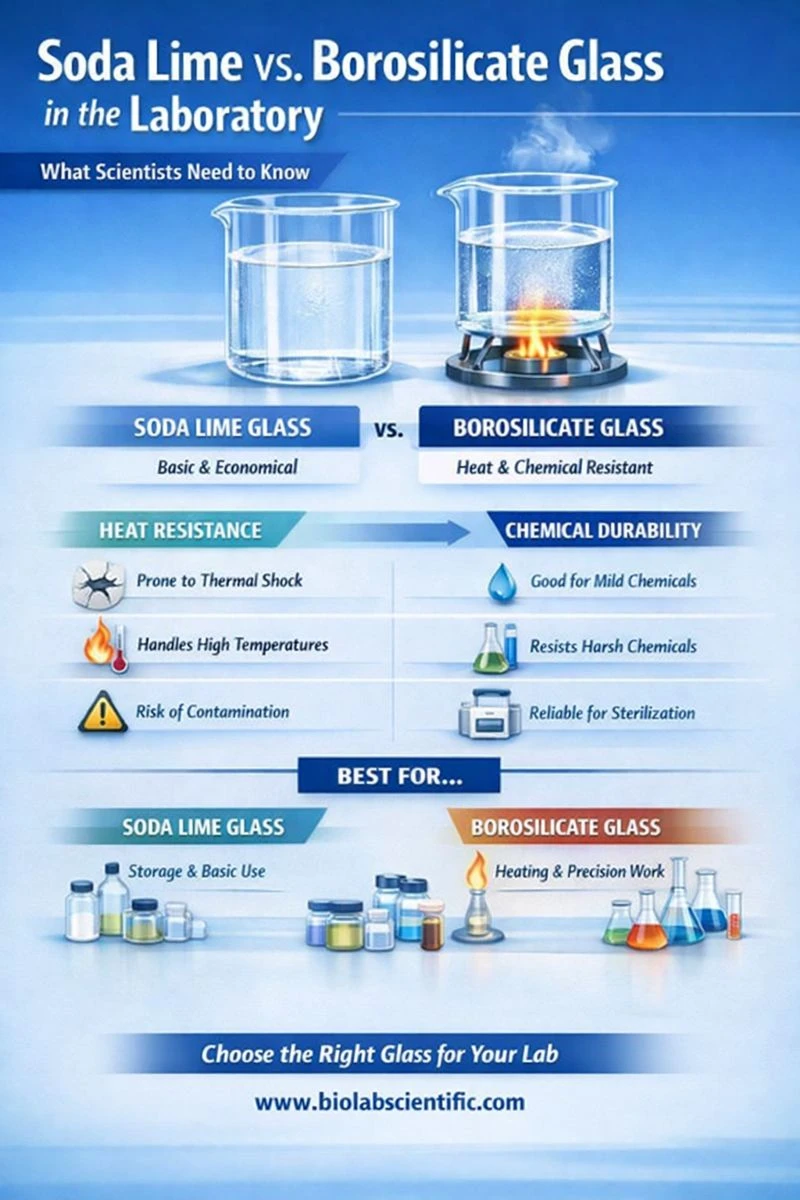
Soda lime glass is the most common everyday glass. It's affordable and works well for basic containers and simple lab tasks, but it does not handle sudden temperature changes or repeated high-heat cycles very well.
Borosilicate glass is a lab-grade glass made to handle heat and chemicals better. It resists thermal shock (rapid heating/cooling) and is more stable for routine lab heating, autoclaving, and chemical work.

Soda Lime vs. Borosilicate Glass
If you only remember one thing:
Soda lime = general purpose
Borosilicate = lab-grade performance
Why They Behave Differently
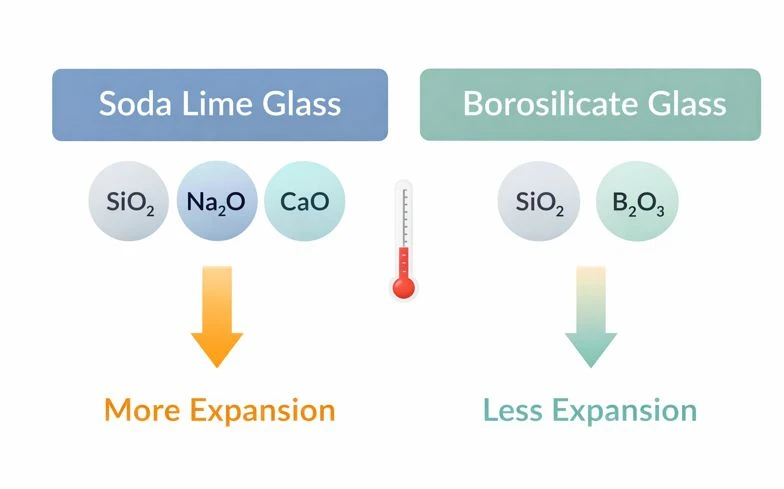
Soda lime and borosilicate glass may look the same, but they act differently because their ingredients are different. Soda lime glass is made mainly with silica + sodium + calcium, which keeps it low-cost and easy to manufacture, but it also makes the glass expand and contract more when temperature changes. That extra movement is why soda lime glass is more likely to crack when it goes from hot to cold (or cold to hot) too quickly.
Borosilicate glass includes boron in the mix, and that changes everything. It expands much less when heated, so it handles rapid temperature changes better and stays more stable during repeated heating, cooling, and sterilization cycles. It also tends to be more chemically resistant, which reduces the risk of the glass slowly reacting with strong chemicals over time.
That's why one type survives a hot to cold temperature change and the other may crack.
Thermal Expansion
Fast Comparison Table
Feature | Soda Lime Glass | Borosilicate Glass |
Best for | Basic lab use, storage, general containers | Heat work, sterilization cycles, sensitive lab work |
Heat resistance | Moderate | High |
Thermal shock resistance | Lower | Higher |
Chemical resistance | Good for mild chemicals | Stronger overall resistance in lab conditions |
Sterilization suitability | Limited / depends on cycle and product | Often suitable / depends on product rating |
Long-term stability for sensitive work | Moderate | Better choice in many labs |
Typical cost | Lower | Higher |
Selecting the Right Laboratory Glass Type
Core Differences That Matter in Real Lab Work
1) Heat and Thermal Shock
This is the biggest day-to-day divider in real labs.
Borosilicate is made for heat stress. It has much better resistance to thermal shock, which means it can handle quick temperature changes without cracking as easily.
That's why it's commonly used for:
Heating on hot plates and stirrers
Boiling, reflux, and water/oil bath work
Moving between warm and cool environments
Regular sterilization and drying routines (within the glassware's rated limits)
Soda lime glass is more sensitive to temperature shock. It can work fine for room temperature tasks, but it's more likely to fail when the lab routine includes repeated heating and cooling. Soda lime tends to struggle when:
You heat it directly on a hot plate
You cool it quickly after heating (cold bench, water rinse, cool air)
You run repeated high temperature cycles (autoclave + oven + wash cycles)
What this looks like in real life: A Soda Lime bottle might survive one or two heating events, then suddenly crack on the third run especially if it already has tiny scratches or micro-cracks. Borosilicate usually gives you a wider safety margin and longer usable life in heated workflows.
Simple rule: If your work includes rapid temperature changes, boiling, frequent sterilization, or drying cycles, borosilicate is usually the safer choice.

Glassware Heating On Hot Plates
2) Chemical Resistance and Sample Safety
Both glass types can handle many mild lab chemicals, but borosilicate is typically preferred when samples are sensitive or reactions are demanding.
Borosilicate is often chosen for:
Analytical work where repeatability matters
Reactions involving acids, solvents, or stronger reagents
Longer storage of chemicals
Work where you want lower risk of ions leaching into samples

Quality Control Standards in Borosilicate Glass
Soda lime is often fine for:
Basic storage (especially short-term)
Non-critical mixing at room temperature
Water-based solutions with mild chemistry
General-purpose containers where small changes won't affect results
Why this matters:
Some tests are very sensitive. Small amounts of ion leaching (like sodium or calcium) can shift pH, affect conductivity, or interfere with trace level measurements. It's not always visible, but it can show up as why are my results drifting? problems.
Simple rule: For routine storage and simple handling, soda lime may be enough. For sensitive samples, stronger reagents, or analytical accuracy, borosilicate is the safer option.
3) Durability in Daily Handling
This is where many labs get surprised.
Soda lime can feel tough in casual use, but it doesn't like repeated heat stress. It may be fine on the shelf, but it's more likely to crack over time if it becomes part of a workflow that includes heating, cooling, autoclaving, and drying.
Borosilicate is built for lab stress, but it still breaks if dropped no glass is drop proof. Its advantage is not unbreakable, it's more reliable under heat and repeated cycles.
The hidden durability issue: scratches
Scratches are a major reason glassware fails early. Once glass is scratched:
It becomes weaker and cracks faster
It traps residues and is harder to clean fully
It's more likely to fail during heating or autoclaving
Small scratches can turn into sudden breakage during the next cycle
Practical ways labs extend glass life:
Avoid metal brushes that scratch inner walls
Don't stack glass-on-glass without protection
Use racks and separators during washing/autoclaving
Discard visibly scratched glass used for heating/sterilization
Don't use chipped rims or cracked bases one more time
Simple rule:If glassware is part of a repeated wash + heat + sterilize routine, borosilicate usually lasts longer and fails less often.
Fast Comparison Table
Feature | Soda Lime Glass | Borosilicate Glass |
Best for | Basic lab use, storage, general containers | Heat work, sterilization cycles, sensitive lab work |
Heat resistance | Moderate | High |
Thermal shock resistance | Lower | Higher |
Chemical resistance | Good for mild chemicals | Stronger overall resistance in lab conditions |
Sterilization suitability | Limited / depends on cycle and product | Often suitable / depends on product rating |
Long-term stability for sensitive work | Moderate | Better choice in many labs |
Typical cost | Lower | Higher |
Common Mistakes That Cause Breakage or Bad Results
Even good glassware fails early when it's used in the wrong way. Most problems come from small habits that seem harmless until a bottle cracks mid-cycle or results start drifting for no clear reason.
1) Heating soda lime glass like borosilicate
A common mistake is placing soda lime beakers or bottles on hot plates, in ovens, or into repeated autoclave cycles. Soda lime expands more with heat, so it's much more likely to crack especially after a few cycles when tiny stress marks build up.
2) Fast temperature changes (thermal shock)
Moving glass from hot to cold too quickly is one of the fastest ways to break it. Examples include rinsing a hot flask under cold water, putting hot glass on a cold metal bench, or taking glass straight from an oven into cool room air. Even borosilicate has limited rapid changes that still add stress.
3) Over-tightening caps before sterilization
Tight caps trap pressure. In an autoclave, that pressure can cause bottles to leak, crack, or even pop open. For liquid media or buffers, caps should usually be loosened (or vented) as per SOP, then tightened after cooling.
4) Using scratched, chipped, or etched glass for heated workflows
Those scratches and chips aren't just about appearance , they're weak points. Damaged glass is much more likely to crack or break when you heat it, autoclave it, or handle it around centrifuges. Cloudy or etched glass can also hold onto residues and interfere with sensitive tests.
5) Stacking glassware without protection
Glass-on-glass contact during storage, washing, or autoclaving creates micro-chips that you may not notice immediately. Over time, those tiny chips turn into cracks or sudden breakage during the next heat cycle.
6) Using the wrong cleaning tools and chemicals
Metal brushes, abrasive pads, or harsh cleaners can scratch the inner surface and reduce the glass life quickly. Strong alkalis and long soaking times can also speed up etching, especially when repeated week after week.
7) Treating general-use glass as analytical glass
For sensitive measurements, contamination isn't always visible. Using the wrong glass can introduce tiny ions (like sodium) into samples, shift pH slightly, or interfere with trace-level testing. The result often appears as drift, inconsistency, or poor reproducibility.
8) Ignoring early warning signs
Cloudiness, dull/frosted surfaces, sticky residues that don't clean easily, hairline cracks, or rough rims are all signs the glass is nearing failure. In cleanrooms and regulated labs, replacing early is usually cheaper than losing a batch, repeating work, or risking contamination.

Safe Glassware Handling Guide
Choosing the Right Glass
In the end, soda lime and borosilicate glass are not competitors, they're tools for different jobs. Soda lime glass is perfectly fine for simple storage, short-term, low-stress tasks and non-critical solutions. Borosilicate glass is the better choice whenever heat, autoclaving, aggressive chemicals or repeated cycles are involved, especially in QC, pharma and cleanroom style environments where even small failures can have big consequences.
Choosing the right glass type protects your samples, reduces breakage, and makes your day-to-day work safer and more predictable. If you'd like help matching glassware to your applications or autoclave cycles, our lab equipment team is available to support you Contact us through our Website to identify the most suitable options for your lab.
You May Also Like