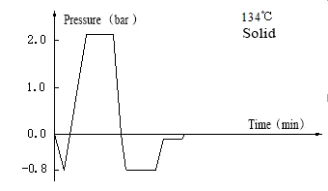
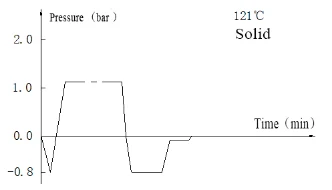
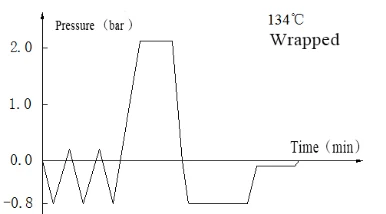
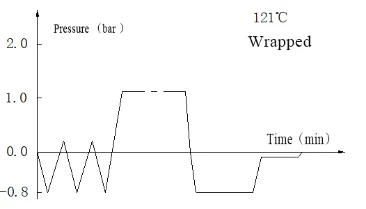
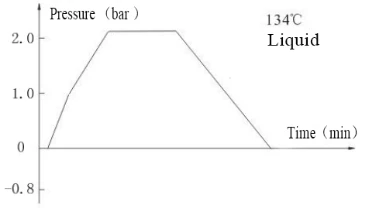
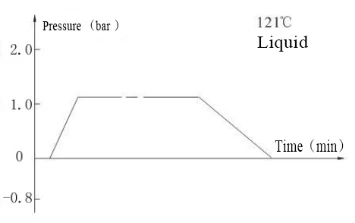
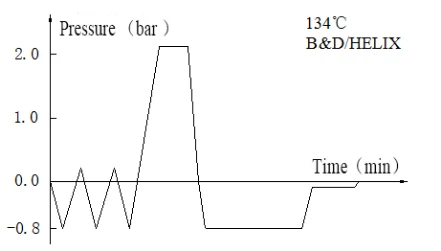
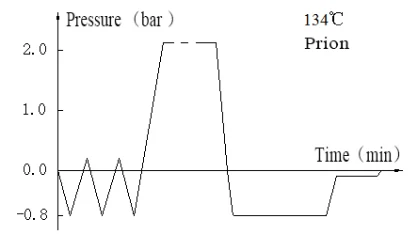
Laboratory Vertical Autoclave BJT1B1 (BAVT-2901)
Vertical Autoclave, Vertical Steam Sterilizer- Sea, Air, Door to Door Shipping
- 1 Year Warranty
- US & European Standards

Specifications
| Model | BJT1B1 |
| Capacity | 35 L |
| Power | 3.5 kW |
| Voltage | AC 220V. 50HZ |
| Design pressure | 0.25 Mpa |
| Design temperature | 139°C |
| Rated working pressure | 0.22 Mpa |
| Rated working temperature | 134°C |
| Sterilization temperature setting range | 116°C~134°C |
| Sterilization time setting range | 4 ~ 120 min |
| Drying time setting range | 0 ~ 240 min |
| Chamber volume | Ø 350x400 |
| Bucket dimension | Ø 330x320 |
| Basket dimension | Ø 320x350 |
| Outer dimension | 698x498x940 |
| Packing size | 790x610x1130 |
| Net weight | 115 Kg |
| Gross Weight | 130 Kg |
Features
Three times pre-vacuumHigh quality stainless steel material
Microcomputer automatic control, arbitrary setting sterilization parameters
Safety interlocking device
LED touch screen display
Standard test interface
Self-expanding seal
With drying function
With stainless steel bucket or basket
With automatic protection function/over temperature protection; over pressure self discharge protection ; low water level protection; anti dry burning.
Automatic water
External steam Generator
Buzzer reminder after sterilization, automatic stop.
Automatic discharge of cold air, automatic steam exhaust after sterilization
Internal printer
Operating Manual
Download0.1 Introduction
0.2 Safety Tips
I. Introduction
III. Technical parameters
IV. Installation requirements
V. Methodology of use
Thank you for choosing our products. Please read the "Operating Manual" before operating this unit to assure proper operation.
Version:A2/20240104
 Warning: To ensure the safe and correct use of the equipment, please read this instruction carefully before use and follow the instructions. Failure to use the method specified by the manufacturer may damage the protection provided by the equipment.
Warning: To ensure the safe and correct use of the equipment, please read this instruction carefully before use and follow the instructions. Failure to use the method specified by the manufacturer may damage the protection provided by the equipment.
0.1 Introduction
Welcome to use the vertical steam sterilizer (hereinafter referred to as sterilizer or machine or equipment).
This instruction describes the operation steps of the equipment. In order to ensure the correct use of the equipment, please read this instruction carefully before using it.
Our company guarantees the quality of the product, according to the relevant regulations of the state and industry to implement the "three guarantees "(except for easily damaged parts) for one year, bear the responsibility directly related to the quality of the product.
Users in the use of any circumstances, can ask our company, we will provide you with warm service.
The company undertakes to provide users with the necessary information about sterilizer maintenance if necessary.
0.2 Safety Tips
(1) Please read this instruction carefully before use and understand all warning and attention requirements. The user must check the safety performance of the sterilizer to ensure that the sterilizer is in good working condition before use.
The sterilizer shall be used in accordance with the scope of application, methods of use and precautions specified in this specification. Otherwise, it may damage the protection provided by the sterilizer or cause sterilization failure.
(3) The equipment is supplied with a number of safety devices to prevent injury to the operator and damage to the equipment, and the operator shall understand these provisions before operating.
(4) Requirements for operators: operators must be trained in equipment performance characteristics, working principles and on-site operation (use) before taking up the post, and have certain knowledge of sterilization technology.
(5) The equipment is a type of pressure vessel and shall comply with the relevant provisions of the national pressure vessel regulations in the course of its use.
(6) We have fully considered the safety of the use of the product in the design and manufacture process, but the operator can not be ignored, and the working status of the equipment should be checked and observed frequently during the use process.
(7) The connection of the network power supply and the power supply used by the user shall comply with the relevant requirements of the national electrical safety standards. When the voltage fluctuation of the power network exceeds 10, the equipment may not work properly.
(8) The equipment meets the requirements of the GB/T 18268.1-2010. It is recommended
that users evaluate the electromagnetic environment before use to ensure that the sterilizer works properly.
(9) The equipment meets the relevant safety requirements of the GB 4793.1-2007,GB 4793.4-2007.
(10) Pressure gauges, safety valves and other instruments shall be regularly identified in accordance with relevant State regulations.
(11) Before installing a fuse or performing electrical maintenance, be sure to cut off the equipment and cut off the total power supply.
(12) To ensure safe use and avoid electric shock injury, ensure that the equipment is well grounded. Please do not change the internal or external grounding protection line of the equipment or remove the connection of the grounding protection section. Otherwise, the protection function of the equipment will be invalid and electric shock will occur.
(13) Be careful and keep away from the ironing area of the equipment to avoid scalding.
(14) When the equipment fails, the total power supply of the equipment shall be disconnected immediately and then connected after troubleshooting.
(15) The accessories of equipment and equipment shall be used during the specified useful life period, and late use may pose certain safety risks. Therefore, its safety should be checked before each use, and should be replaced in time if necessary
(16) If there is interference in the power grid, there may be a white screen of the LCD screen when the equipment is used, and the users who have this problem can be equipped with a voltage regulator to solve the above problem.
I. Introduction
The vertical steam sterilizer is a kind of equipment that uses saturated steam to sterilize medical devices, dressings, medicine liquid, glassware and so on.It has the advantages of novel and beautiful shape, reasonable structure, complete function, rapid heating and thorough sterilization.
Product intended use: for clinical institutions for medical devices, dressings, glassware, solution medium steam disinfection sterilization.
Product taboo: none.
Intended locations for use: medical and health institutions, operating rooms, laboratories, etc.
Applicable crowd: medical and health unit staff.
II. Structural characteristics
1. Vertical steam sterilizer is mainly composed of shell, sterilizing chamber, steam generator, pipeline system and control system. The structure of vertical steam sterilizer is divided into vertical type. The functional design is divided into: automatic control type (B type), automatic drying type (G type), automatic drying internal circulation type (X type), pulsating vacuum type (M type). The container seal structure is divided into beam type (H), bolt type (S), flip type (F). Press sterilizing chamber volume integral :30 L,35L,40L,50L,60L,75L,80L,100L,120L and 150 L several models.
2. Shape is square vertical structure, sterilizer shell, sterilizing chamber, sterilizing basket is made of stainless steel material. Sterilization rooms (pressure vessels) and their safety accessories shall comply with the regulations on the Safety Supervision of GB 150,《 Special equipment and the national statutory pressure vessel regulations, There should be a statutory pressure vessel safety supervision agency registered product nameplate and product quality certificate.
3. Fitted with safety valves. If the pressure in the vessel exceeds 0.24 MPa, the safety valve can automatically release excessive pressure, which is safe and reliable.
4. During heating and heating, the vent valve will open and automatically discharge cold air. If the temperature exceeds 102°C, the valve will automatically close and then switch to the automatic intermittent degassing function to improve the steam saturation in the container and the sterilization effect will be better.
5. Operation panel is equipped with liquid crystal display screen, with pressure, temperature, time, flow indication, clear observation.
6. This container has the function of automatic protection, when the container is short of water, the program can not start, effectively protect the use of equipment, and the water level icon shows low water level or water shortage to remind users to replenish water in time.
III. Technical parameters
No | Volume | Max.working pressure | Max.sterilization Working temperature | Sterilization Time | Power |
1 | 35L | 0.22 MPa | 134°C | 4~120 min | 3.0KW |
2 | 50L | 3.5KW | |||
3 | 75L | 4.5KW | |||
4 | 100L | 4.5KW |
Table 1
1. Power supply: AC220V/50H z or AC110V/60Hz
2. Working pressure :0.22 MPa; Working temperature :105°C~134°C.
3. When it is in normal operation during the normal sterilization cycle, the noise should not be greater than 65 dB (A weight).
4. Set sterilization time range :4~120 minutes; set drying time range :0~240 minutes.
5. Equipment safety category: I class.
6. Ambient temperature :+5°C~+40°C; relative humidity: not greater than 85; atmospheric pressure 70 kPa°C~+106
7. Water sources used in sterilizers should not affect the sterilization process and damage sterilizers or sterilizers.
IV. Installation requirements
1. Before use, please read this manual carefully.
2. This equipment according to electric shock protection class I, the installation site should not be flammable and explosive anesthetic gas and articles.
3. The sterilizer is permanently connected to the grid, The user must be 60 cm from the building, Height greater than 1.2 m, The circuit breaker (which shall meet the requirements of the GB 14048.1 and GB 14048.3 standards) is used as the disconnecting device for the power supply of the sterilizer, And do a good job of obvious on-off signs (such as sterilizer special). The power cord should be fixed on the building before it is connected to the circuit breaker to avoid falling off, Connection line diameter 2.5 mm²x3, L is the line of fire, N zero, Yellow and green two-color G for protection grounding. After work, Shut down the breaker.
4. The ground of this equipment must be flat and firm. According to the parameters specified in the product nameplate and specification, the equipment shall be connected to the specified power supply according to the circuit wiring diagram, and the equipment shall be reliably grounded (the grounding resistance value shall not exceed 2Ω).
5. Do not enter the drainage end of the drain pipe into the water to prevent the water from being sucked into the sterilizer barrel.
V. Methodology of use
The pressure vessel random documents shall be submitted to the local supervision and inspection agency for registration and record before the sterilizer is used.
The operator of the sterilizer must be professionally trained, familiar with the operating essentials of the pressure vessel and operate strictly in accordance with the instructions.
Sterilizer should be supervised by professional personnel and make a good record of sterilizer operation to prevent accidents or accidents.
The safety valve of the sterilizer is sent to the inspection organization at least once a year, and the test is qualified to continue to be used.
Danger:
Do not place combustible substances: do not place or approach the following substances specified in item 1 of the Schedule to the Labor Safety and Health Act :(explosive, flammable, oxidizing, igniting, combustible) or cause injury, fire or malfunction due to explosion.
Note:
- Do not put corrosive stainless steel substances: do not corrosion stainless steel medium and so on into this product. Otherwise, it will cause injury, fire or malfunction due to explosion.
- Do not touch power switches and circuit breakers with wet hands to avoid electric shock and accidents.
- The container cover of the sterilizer is in high temperature in working condition and is forbidden to touch.
-"If the steam is not exhausted, it must not be opened ": after sterilizing , when the pressure gauge does not return to zero, the container cover shall not be opened, otherwise it will easily cause high temperature steam burns.
(i) the symbols and meanings of the safety requirements in this machine
Symbol | Meaning | Symbol | Meaning |
~ | AC | | Attention! Access to random documents |
| Be careful | | Protection ground |
| Test interface |
Table 2
(ii) Operations nal ste
1. Ready
1.1 Opening of sterilizer doors
1.2 Connect sterilizer power
1.3 Connect the inlet to the pure water source and the drain to the sewage discharge(Do not come into contact with sewage)
1.4 Turn on the power, press the switch "I" power on,
 ----------------------This icon is displayed when the door is not closed
----------------------This icon is displayed when the door is not closed
 ----------------------This icon is displayed when the door is closed
----------------------This icon is displayed when the door is closed
2. Select program and Parameter settings

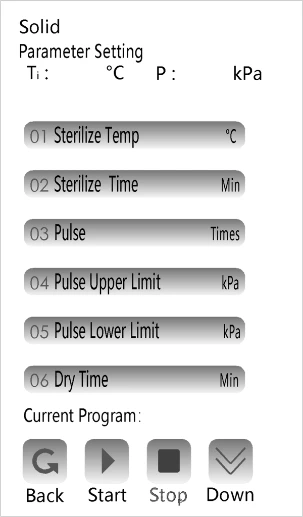

Figure 1
Touch the numeric keypad that needs to be changed, touch the "OK "to save .
Note: The user can change the sterilization temperature and time and drying time by entering the technologist interface. The operator cannot make any changes to it after the changes are completed.
Other parameters have been changed in place according to the corresponding program differences before delivery (※ Users are not recommended to set by themselves). If users have requirements, they can choose "Custom program" (the parameters in this program are adjustable by users, please consult the technical personnel of the manufacturer to confirm the feasibility of parameters before adjustment).
3. Program start and stop
After checking whether the water source access is reliable, then you can press the "  "
"
to start the selected program. (If you do not select this option, the system starts the current
program by default.)
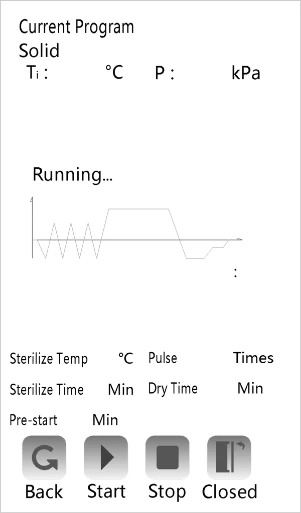




Figure 2
This equipment is automatic water inlet type, start the evaporator to start water (ensure reliable water connection).
After sterilization, the printer will print automatically. If sterilization process parameters need to be saved, insert the USB flash drive before the end of the procedure.
4. System parameter setting (user setting is not recommended)
Note:
Sterilization temperature correction is related to the performance of equipment, users should not easily change or set
If you need to adjust the parameters, you can go to the administrator interface and perform the following steps to adjust the parameters.
Press the " ",input the password"5566"then into the System Parameter interface
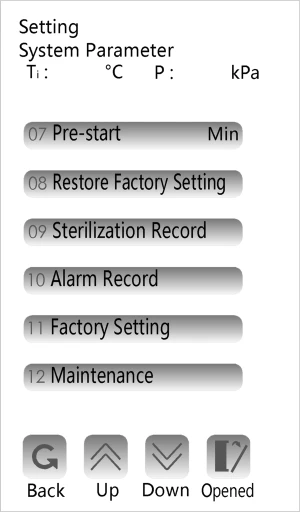


Figure 3
Note:
Altitude offset(±90°C)
Inner temperature offset (±10°C)
Inner pressure offset (±30 Kpa)
(iii) Preparation of instruments
A. Stacking
(1) Turn the hand wheel several times counterclockwise (note that the hand is clamped and cause injury) until it is turned to the top so that the sterilizer cover is fully lifted and the crossbeam is pushed to the right to remove the sterilizer cover. Take out the sterilizing basket or bucket.
(2) This product is automatic adding water. The distilled water source that meets the requirements will be directly connected to the inlet.
(3) The articles to be disinfected shall be properly bandaged and placed in a sequential sterilization basket with a gap between them. This is conducive to steam penetration and improve sterilization effect. When stacking sterilizing bags, attention should be paid to leaving the gap of the relief valve outlet hole, otherwise, the pressure can not be released due to the blockage of the relief valve hole, resulting in unnecessary personal equipment accidents.
B. Power Switch on the power consistent with the sterilizer label, press the switch on the control panel to "I", and the screen will be lit up.
C. Function setting User according to different sterilization items select the required key to set the required sterilization temperature, time. (See operating panel description)[ Sterilization temperature and sterilization time for different items, refer to the Ministry of Health Technical Specification for Disinfection. ]
D. Seal Pushes the beam to the left column and rotates the handwheel clockwise to press the sterilizer cover and the lower flange. When the LCD displays the door closed, it should continue to rotate until it is completely closed. )
E. Heating Press start button to start the program.
F. Sterilization Temperature rises to the set temperature, start sterilization.
G. Exhaust steam (no use of this method when sterilizing liquid)
After sterilization, start automatic exhausting steam.
H. Drainage This product is water circulation and with water quality detection function, after sterilization without manual drainage. After the water quality reaches the alarm value,it is necessary to replace the water source and first close the machine power supply, open the left door, open the internal evaporator drain valve and the outer water storage tank drain valve to drain the water.
I. Drying After sterilization, start automatic drying.
J. End Panel display window shows "end" and has a prompt tone indicating end. Remove items by opening the cover (please be sure to wear protective gloves to remove.It will take some time for the temperature to drop, so please take it out carefully.Burns may be caused by direct touching of hands after operation)
Remarks
1.In the course of running the program, if there is an alarm sound and a warning symbol is displayed ,(the meaning of the symbol is detailed in the common fault code of this manual), the error is indicated.
I2.f you find that the selection settings are wrong after running, you can press the stop button to interrupt the program.
3.If the error code is displayed in the upper left corner of the screen, You can touch the code to cancel the error code(When the pressure drops to ±5 kPa, you can cancel the error code.)
4.Evaporator water level float ball shall be cleaned regularly.
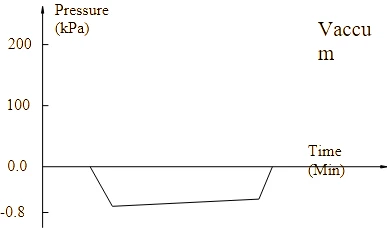
Program Diagram
|
|
|
|
|
|
|
|
| |
Table 3
Program operation flow:
Start -- pulsating vacuum -- heating -- sterilization -- exhaust -- pulsating vacuum drying -- end
IV. Attention And Maintenance
Note :1.Please ensure the EMC environment before use, so that the equipment works properly.
2.Recommend that users evaluate EMC before the device is used to ensure that the instrument works properly.
3.Prohibit the use of this instrument next to a strong radiation source, otherwise it may interfere with the normal opera.
1. Stacking sterilizing articles (completely opening the upper cover), the basket should be placed in the center of the barrel (the basket and basket should be concentric, stacked up and down). Otherwise, the safety valve can not work because of the hole blockage, resulting in accidents.
2.When the sterilizer continues to work, leave 5 minutes for new sterilization operations and open the upper cover to allow the equipment time to cool.
3.When running the "liquid sterilization" program, the liquid should be canned in a hard heat-resistant glass bottle, not more than 3/4 volume, the bottle mouth cotton yarn plug, do not use unperforated rubber or cork.
Special note: Do not release steam immediately at the end of sterilizing liquid, must wait until the pressure gauge pointer returns to zero before discharging residual gas.
4. Only the beaker, flask, test tube and other containers for sterilization treatment, its opening must be placed face down or horizontally. When the opening part is placed upward, it is not only difficult to deflate, but also not easy to infiltrate steam into its interior, which leads to the appearance of poor sterilization effect.
5. For different types, different sterilization requirements, such as dressings and liquids, do not put together to sterilize, lest cause loss.
6. Confirm the sterilization performance: the sterilization performance is different due to the type, quantity, put-in method, container type, so please use the sterilization indicator of OK card and so on to confirm. Otherwise, it will lead to accidents or poor sterilization effect.
7. Do not leave sterilized articles in this product: This product has no retention function for sterilized items. In order to avoid contamination again, after sterilizing the medium and utensils, please take them out immediately and keep them in a special safe.
8. At the end of sterilization (or in case of failure), the pressure must return to zero or turn off the power . Make the outside air into the sterilizer, after vacuum elimination, the container cover can be opened (Keep your face away from the cover), after the cover is completely opened, take out the basket to pay attention to scalding and clamping. Be sure to wear protective gloves for removal. Due to the drop of liquid temperature will take some time, please be careful when taking out. After running, touching directly with your hand may cause burns. )
9.Apply proper amount of high temperature grease to the beam rod every six months.
10. The equipment should be kept clean and dry at ordinary times. Rubber seal gaskets will age for a long time and should be replaced regularly (recommended 12 months).
11. In use, when the pressure indication exceeds 0.24 MPa, the safety valve does not start, the power supply should be cut off immediately, after 10 minutes, manually open the safety (lift the safety valve cap), until the steam in the sterilizer is completely released before opening the container cover and replacing the safety valve in time.
12.When troubleshooting requires replacement of components, there must be professionally trained qualified personnel or factory personnel. The power circuit breaker is disconnected, the remaining gas in the vessel is exhausted, the pressure gauge pointercan be returned to zero, and the safety valve is submitted for inspection at least once a year.Put up and down the safety valve several times a week to prevent the spool from scaling and leaking.
13.Do not alter use: do not break down or repair without authorization, except for maintenance technicians. Otherwise, it will be injured by fire or abnormal operation.
14. Cleaning (recommended once a month): water should be emptied and power should be cuted off. After the container is cooled, remove the shelf and basket from the container, first use a brush to attach to the inner wall, then dip the towel with alcohol or distilled water, wipe the inner surface, after drying, put the shelf and basket in turn. Store in a non-corrosive gas and well ventilated room.
15.The interlocking device of the door cover consists of a self-locking device and a gating switch. The expansion and flexibility of the locking tongue of the self-locking device should be maintained in daily use (quarterly inspection is recommended). Before the pressure in the vessel is released to zero, the container cover shall not be forcibly opened, otherwise the self-locking device will be damaged. Gated switch should keep the contact up and down flexible, when the contact card rolling and indicator icon abnormal work, should be timely maintenance.
16. Sterilizers shall not be used by non-operators: otherwise they may cause burns, electric shocks or injuries.
17.Do not open the drain valve during the operation of the equipment, wait for more than 2 hours after sterilization and perform the drainage operation only when the lid is opened. Otherwise, it will cause burns or accidents due to the injection of high temperature hot water.
18. Replacement of fuses: when the power cord of the sterilizer is connected to the power supply, when the power indicator lamp is not on, the circuit breaker should be disconnected, the back cover of the sterilizer should be opened with a cross screwdriver, and the fuse (specification type R015,10x38,32 A) should be damaged or replaced immediately if damaged.
19. Before moving or transporting or storing, the water in the container should be emptied, the sterilizer cover sealed, the circuit breaker cut off and the power cord removed. When moving (please remove the locking state of the casters first) push the sterilizer flat; transport, store (fix the sterilizer in the packing box) to prevent loose and scratch during transportation. Avoid overturning, resulting in injury or accident.
20. Environmental conditions for transport and storage:
ambient temperature :-40°C~+55°C relative humidity range :10%~80% atmospheric pressure range :500 hPa~1060
21. At the end of the product's service life (8 years)(the date of production is shown in the label), the product and accessories shall be voluntarily handed over to the unit or individual with corresponding qualifications for recycling.
22. Wiring diagrams:
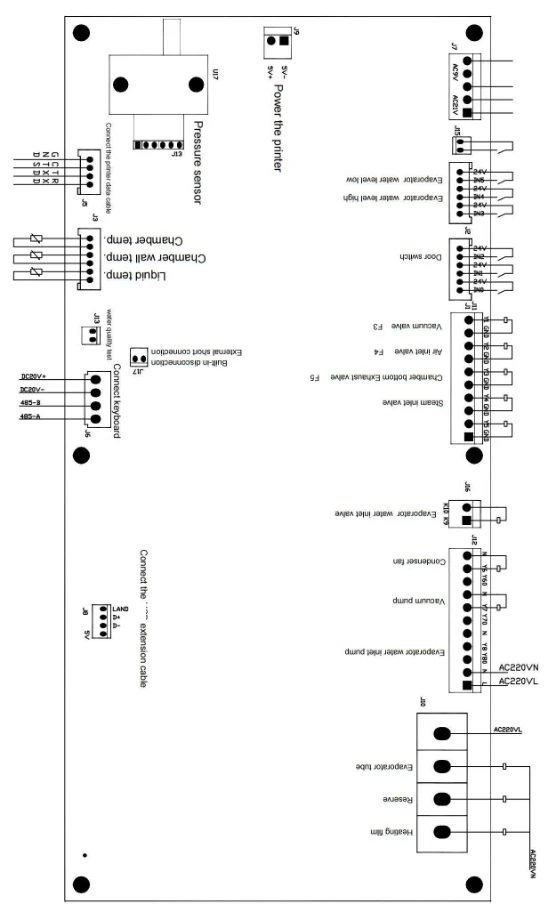
Figure 4
(If necessary, can provide circuit diagram and repair necessary information, electrical line maintenance if there are difficult problems, can contact our company.)
23. Piping diagram
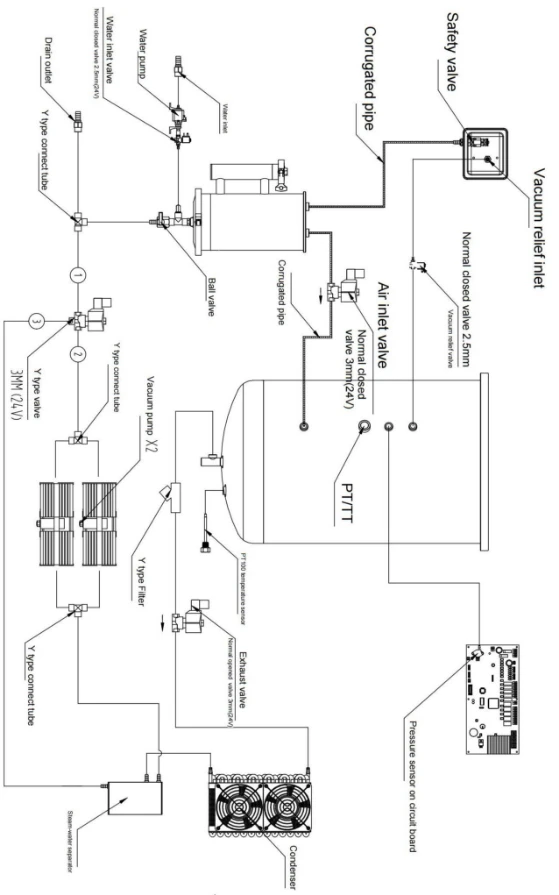
Figure 5
V. Common fault codes
E002 | The sterilization outdoor wall temperature sensor failure(Short circuit: 0 is displayed. Open circuit: 250.0 is displayed) | Check the heating film Check the sensor wiring Replace the outer wall sensor |
E003 | The sterilization of the chamber temperature sensor failure(Short circuit: 0 is displayed. Open circuit: 250.0 is displayed) | Check the PT100 Replace the inner chamber sensor |
E004 | Heating timeout 60min | Check the solenoid valve 2. Check the built-in evaporator heating pipe |
E005 | Pressure rise failure | 1. Check the sealing of the chamber (door, line, solenoid valve) |
E006 | The door closure position disconnected | 1. Check the gate and gating line |
E009 | Pressure holding failure during sterilization (less than 160KPA when greater than 134 degrees, less than 10KPA when less than 121 degrees, and less than 70kpa when the rest) | 1. Check the sealing of the chamber (door, line, solenoid valve) |
E010 | Overpressure and overtemperature alarm (pressure exceeding 233KPa, temperature exceeding setting +4°) | Check whether the exhaust valve works normally |
E012 | Insulation layer heating failure (the temperature did not rise to 50 degrees within 10 minutes during preheating) | Check whether the heating film cable falls off Replace the heating film (by professionals) |
E013 | vacuum failure(preset time during vacuum test: 5 minutes) | Check the sealing of chamber(door, pipeline, solenoid valve) Check the performance of vacuum pump Replace vacuum pump and vacuum valve (professionally replaced) |
E014 | Unexpected power failure during operation | After restart, press the alarm code to cancel |
E015 | The working process is interrupted | After the alarm sound is over, Press the alarm code to cancel |
E016 | The sterilization indoor pressure sensor failure | Check and replace the circuit board. Check the pipe |
E017 | Into the water failed | 1.Whether the water pump works normally. 2.Contact a professional for troubleshooting |
E019 | Water level fault | Check the water level float Needs to be cleaned |
E020 | Communication failure | Check the wiring |
Table 4
Packing list:
NO | Name | Quantity | Remarks |
1 | Vertical steam sterilizer | 1 | |
2 | Operating manual | 1 | |
3 | Basket or bucket | 2 or 1 | |
4 | Feed water pipe | 1 | 2 m length |
5 | Drainage pipe | 1 | 2 m length |
6 | Filter of feed water pipe | 1 |
Table 5
EMC requirements:
Appendix VI
EMC requirements:
Guide and manufacturer's statement -- electromagnetic emission | ||
The desktop sterilizer is expected to be used in the following specified electromagnetic environment, and the purchaser or user shall ensure that it is used in such electromagnetic environment: | ||
Launch test | Conformity | Electromagnetic Environment -- Guide |
RF GB4824 | Group 1 | Desktop sterilizers use RF energy only for their internal functions. Therefore, its RF emission is very low and the possibility of interference with nearby electronic devices is very small |
RF GB4824 | B category | Desktop sterilizers are suitable for use in all facilities, including household facilities and public low-voltage power supply networks directly connected to residential homes |
Harmonic Emission GB17625.1 | A category | |
Voltage fluctuation, flicker emission GB17625.2 | Compliance | |
Table 6
Guide and manufacturer's statement -- electromagnetic immunity | |||
The desktop sterilizer is expected to be used in the following specified electromagnetic environment, and the purchaser or user shall ensure that it is used in such electromagnetic environment: | |||
Disturbance test | IEC60601 test level | Level of compliance | Electromagnetic Environment -- Guide |
Electrostatic discharge GB/T17626.2 | ±4 kV Contact discharge ±4 kV Air discharge | ±4 kV Contact discharge ±4 kV Air discharge | The floor shall be wood, concrete or ceramic tile, and the relative humidity shall be at least 30% if the floor is covered with synthetic material |
Electric Fast Instantaneous Pulse Group GB/T17626.4 | ±2 kV Power cord ±1 kV to input/output lines | ±2 kV Power cord Not applicable | Power supply should be of typical commercial or hospital quality |
Surge surge GB/T17626.5 | ±0.5 kV line alignment ±1 kV Line to ground | ±0.5 kV line alignment ±1 kV Line to ground | Power supply should be of typical commercial or hospital quality |
Voltage sag on power input line, short time interrupt and voltage change GB/T17626.11 | ﹤5%UT For 0.5 weeks (at UT﹥95% the temporary drop) 40% UT40 5 weeks (at UT 60% the temporary drop) 70 %UT For 25 weeks (at UT ,30% the temporary drop) ﹤5UT For 5 s (at UT﹥95 %the temporary drop) | ﹤5%UT For 0.5 weeks (at UT﹥95% the temporary drop) 40% UT40 5 weeks (at UT 60% the temporary drop) 70 %UT For 25 weeks (at UT ,30% the temporary drop) ﹤5UT For 5 s (at UT﹥95 %the temporary drop) | Network power should have the quality of use in a typical commercial or hospital environment. If the user of the desktop sterilizer needs continuous operation during the power outage, it is recommended that the desktop sterilizer be powered by an uninterruptible power supply or a battery |
Power frequency magnetic field 50Hz GB/T17626.8 | 3A/m | 3A/m | The power frequency magnetic field should have the power frequency magnetic field horizontal characteristic in the typical commercial or hospital environment |
Note1:UT Refers to the AC network voltage before the test voltage is applied | |||
Table 7
Guide and manufacturer's statement -- electromagnetic immunity | |||
The desktop sterilizer is expected to be used in the electromagnetic environment specified below, and the purchaser or user shall ensure that it is used in this electromagnetic environment. | |||
Disturbance test | IEC60601 test level | Level of compliance | Electromagnetic Environment - Guide |
RF conduction GB/17626.6 Radio frequency radiation GB/17626.3 | 3V (valid values) 150 kHz ~ 80MHz 3V/m GHz 80MHz ~2.5 | 3V( valid values) 3V/m | Portable and mobile RF communication equipment shall not be closer to any part of the desktop sterilizer (including cables) than the recommended isolation distance, which shall be calculated using a formula corresponding to the frequency of the transmitter. recommended isolation distance d =1.2 d =1.2 MHz 80MHz ~80 0 d =2.3 GHz 800MHz ~2.5 The maximum output power of the transmitter, in watts (W), is P- provided by the transmitter manufacturer d--- recommended isolation distance, in meters (m); and The field strength of the fixed RF transmitter, determined by the survey of the electromagnetic field, should be lower than the conformance level in each frequency range. Interference may occur near the device that marks the following symbols. |
Note 1: the formula of higher frequency band should be used in 80 MHz and 800 MHz frequency. Note 2: These guidelines may not be suitable for all situations. Electromagnetic propagation is affected by the absorption and reflection of buildings, objects and human bodies. | |||
:: Fixed transmitters, such as radio and ground mobile radio base stations, amateur radios, AM and FM radio broadcasts and television broadcasts, whose field strength is theoretically unpredictable. In order to evaluate the electromagnetic environment of fixed RF transmitter, the survey of electromagnetic field should be considered. If the field strength of the place where the desktop sterilizer is measured is higher than the above RF conformance level, the desktop sterilizer should be observed to verify its normal operation. If abnormal performance is observed, supplementary measures may be necessary, such as readjusting the direction or location of the desktop sterilizer. :: The field strength should be less than 3 V/m. MHz the full frequency range of 150KHz~80MHz | |||
Table 8
Recommended isolation distance between portable and mobile radio frequency communications equipment and desktop sterilizers | |||
Desktop sterilizers are expected to be used in controlled electromagnetic environments with RF radiation disturbance. Depending on the maximum output power of the communication device, the purchaser or user of the desktop sterilizer can prevent electromagnetic interference by maintaining the minimum distance between the portable and mobile radio frequency communication equipment (transmitter) and the desktop sterilizer | |||
Maximum rated output power of transmitter W | Distance/ m corresponding to different frequencies of transmitter | ||
150 kHz ~80 MHz d =1.2 | 80MHz ~80 0MHz d =1.2 | 800MHz ~2.5GHz d =2.3 | |
0.01 | 0.12 | 0.12 | 0.23 |
0.1 | 0.38 | 0.38 | 0.73 |
1 | 1.2 | 1.2 | 2.3 |
10 | 3.8 | 3.8 | 7.3 |
100 | 1.2 | 1.2 | 23 |
the maximum rated output power of the transmitter not listed in the table above is recommended for isolation distance d, in meters (m), which can be determined by the formula in the corresponding transmitter frequency bar. here P is the maximum rated output power of the transmitter provided by the transmitter manufacturer in watts (W) units. Note 1: at 80 MHz and 800 MHz frequency points, the formula of higher frequency band should be adopted. Note 2: These guidelines may not be suitable for all situations. Electromagnetic propagation is affected by the absorption and reflection of buildings, objects and human bodies. | |||
Table 9
This product will not produce strong electromagnetic wave during operation, but in order to ensure the normal use of other medical devices, try to avoid the use of other highly sensitive instruments and equipment at the same time, so as to avoid the interference of the instrument and equipment by electromagnetic sources.