
pH/Ion Meter BMET-503
- Sea, Air, Door to Door Shipping
- 1 Year Warranty
- US & European Standards
An ion meter is a device which with the aid of electrochemical activity of ion electrodes measures the concentration of ions in a solution. It works by the means of selective ion electrodes and measuring ion activity capability of sensitive
membrane of a measuring devices.
A sensor electrode and a reference electrode are used to measure the ion concentration in a solution.
Specification
Features
| pH: | |
| Range | -2.00 to 20.00 pH |
| Resolution | 0.1, 0.01 pH |
| Accuracy | ± 0.01 pH |
| Calibration Points | Up to 5 |
| Standard Customization | Yes |
| Standard Recognition | NIST, GB and DIN buffers |
| mV: | |
| Range | -2000.0 to 2000.0 mV |
| Resolution | 0.1 mV |
| Accuracy | ± 0.3 mV or ± 0.1 % of reading whichever is greater |
| pX: | |
| Range | - 2.00 to 20.00 |
| Resolution | 0.1, 0.01 pX |
| Accuracy | ±0.02 pX |
| Calibration Points | Up to 5 |
| ISE: | |
| Range | 1E-9 to 9.999E9 |
| Unit | mol/L, mmol/L, g/L, mg/L, μg/L, ppm |
| Resolution | Up to 4 significant digits |
| Accuracy | ± 0.5 % |
| Calibration Points | Up to 5 |
| Temperature: | |
| Range | -5 to 110 °C, 23 to 230 °F |
| Unit | °C, °F |
| Resolution | 0.1 |
| Accuracy | ± 0.2 |
| Measurement: | |
| Reading Mode | AutoRead( Fast, Medium,Slow), Timed, Continuous |
| Reading Prompts | Reading, Stable, Locked |
| Temp. Compensation | ATC, MTC |
| Data Management: | |
| Data Storage | 500 results each |
| GLP Features | Yes |
| Inputs: | |
| pH Electrode | BNC(Q9) |
| Temp./DO Probe | 4-pin aviation connector |
| Outputs: | |
| USB | PC |
| RS 232 | printer |
| Display Options: | |
| Backlight | Yes |
| Auto Shut-down | 1~60 min, off |
| IP Rating | IP54 |
| Date and Time | Yes |
| General: | |
| Power | AC Adapter, 100-240 V AC input, DC 9 V output |
| Dimensions | 242x195x68 mm |
| Weight | 900 g(1.98 lb) |
Operating Manual for BMET-503
1. Introduction
1.1 Introduction
1.2 Technical Specification
1.3 Function Introduction
2. Safety Notices
3. Terms Explanation
4. Overview and Installation
4.1 Overview
4.2 Instrument Installation
5. Instrument Operation
5.1 Switch On/Off
5.2 Screen Icons
5.3 Function Key
5.4 Instrument settings
5.5 pH Measurement
5.6 Ion measurement
5.7 Data Management
6. Maintenance/Troubleshooting
6.1 Meter Maintenance
6.2 Electrodes Maintenance
6.3 Troubleshooting
7. Technical Supports
8. Appendixes
1. Introduction
1.1 Introduction
BMET-503 pH/Ion meter is a newly designed functional instrument, support pH, potential, ion concentration measurement, can be widely used in universities, environmental protection, medicine, food, sanitation, geological prospecting, metallurgy, ocean exploration and other fields measurements for acid rain detection, industrial wastewater, surface water, drinking water, beverages, daily chemical products, textiles and other related industries.• General Features
• High resolution LCD display, 5.7 inches.
• Multi-reading feature allows auto-read, timed-read and continuous-read.
• Automatic/Manual temperature compensation ensures accurate results.
• Auto-hold feature senses and locks the measurement endpoint.
• Data Storage 500 sets (GLP-compliant).
• Support for USB or RS-232 communication.
• Reset feature automatically resumes all settings back to factory default options.
• IP54 waterproof.
pH
• 1-5 points calibration with Standard Recognition.
• Selectable pH buffer groups, including NIST, DIN, GB and USA.
• Automatic electrode diagnosis with pH slope and offset display.
Ion
• 1-5 points calibration.
• Selectable measurement unit, including μg/L, mg/L, g/L, mmol/L, pX, ppm, and ppb.
• Multi-measurement modes are supported, including Direct Reading mode, Standard Addition mode, Sample Addition mode and GRAN mode.
• Over 10 methods are built-in, including F-, Cl-, Br-, I-, NO3-, BF4-, NH4+, K+, Na+, Ca2+, Cu2+, Pb2+, Ag+ and etc., user-defined method is supported.
1.2 Technical Specification
Instrument Specifications| Model | BMET-503 | |
| pH/pX level | 0.01 pH/pX | |
| mV | Range | (-2000.0~ 2000.0)mV |
| Resolution | 0.1mV | |
| Accuracy | ±0.1% or ±0.3 mV | |
| Repeatability | 0.5mV | |
| Input Current | ≤1×10 -12 A | |
| Input Impedance | ≥1×10 12 Ω | |
| pH | Range | (-2.00~ 20.00)pH |
| Resolution | 0.01pH | |
| Accuracy | ±0.01pH | |
| Repeatability | 0.005pH | |
| Measurement Accuracy | ±0.02pH | |
| Measurement Repeatability | 0.01pH | |
| pX | Range | (-2.00~ 20.00)pX |
| Resolution | 0.01pX | |
| Accuracy | ±0.01pX | |
| Repeatability | 0.005pX | |
| Measurement Accuracy | ±0.02pX | |
| Measurement Repeatability | 0.01pX | |
| Ion concentration | Range | (1E-9~9.999E9), mol/L, mmol/L, g/L, mg/L, μg/L, ppm, ppb |
| Resolution | 4 significant digits | |
| Measurement Accuracy | ±0.5% | |
| Temperature | Range | (-5.0~ 110.0)℃ |
| Resolution | 0.1℃ | |
| Accuracy | ±0.2℃ | |
| Instrument indication error | ±0.4℃(0.0℃~60.0℃), ±1.0℃(Else) | |
| Work environment | Ambient temperature: (0~ 40)℃ Relative humidity: not more than 85% | |
| Dimensions (L×B×H), weight (kg) | 242mm×195mm×68mm, 0.9kg | |
| Power supply | Power adapter (Input AC 100~240V, Output DC 9V) | |
Table 1
1.3 Function Introduction
Functions Specification| Features | Explanation | |
| Basic Function | Languages | English |
| Backlight adjustment | ● | |
| Automatic diagnostics | ● | |
| Factory reset | ● | |
| Default parameter | ● | |
| Prompt Sound | ● | |
| Time settings | ● | |
| Power failure protection | ● | |
| Firmware upgrade | ● | |
| Anti-interference automatic recovery | ● | |
| Automatic shutdown | ● | |
| Protection | IP54 | |
| Reading function | Balance condition setting | ● |
| When balance state reached, display the reading stability indicator | ● | |
| End point judgment/reading mode | ● | |
| Sample ID input | ● | |
| Data Management | Storage | 500 sets |
| View | ● | |
| Delete | ● | |
| Data Management | GLP | ● |
| Communications and external devices | Printer | Serial Printer |
| Content and format customization | Standard, GLP, Custom format | |
| Communications and external devices | PC | ● |
| pH/mV Measurement | pH electrode status/performance | Slope |
| Multi-point calibration | 5 points | |
| Automatic standard solutions recognition | 3 groups | |
| Standards customization | ● | |
| Standard groups customization | 1 group | |
| Automatic temperature compensation | ● | |
| Manual temperature compensation | ● | |
| pH electrode diagnostics | ● | |
| pX/ISE Measurement | Multi-point calibration | 5 points |
| Optional units | ● | |
| Measurement mode | Direct reading concentration method | |
| Built-in ions methods | ● | |
| Ions customization | ● | |
| Temperature Measurement | Temperature units | ℃ , ℉ |
Table 2
2. Safety Notices
Please read the entire contents of this manual carefully before use, and please keep this manual properly. The user MUST use the instrument following this manual to avoid damage to the user and equipment.Before using the meter, READ the following notes:
• DO NOT disassemble the device for inspection or repair.
• To prevent electric shock or damage to the device, do not place cables and connectors in any liquid, wet or corrosive environment.
• Please use the defaulted power adapter, Do not use it if the power cord is damaged (the wire is exposed or broken).
• Do not use in flammable and explosive environments.
• Do not use if the user finds any abnormalities such as damage or deformation of the device.
The following identifier will be used in this manual.
 TIPS:
TIPS:Tips help users to use the meter.
3. Terms Explanation
• pH/pX• pH/pX Slope: The amount of potential change generated by each 1 pH/pX change, expressed in mV/pH or by 100% Theoretical Slope (PTS). pX = - log[X], where [X] means molar concentration (mol/L) of X ions.
• E0 of pH: Also known as "zero potential", it usually refers to the potential value at a pH of 7.
• One-point calibration: Calibration with a standard solution.
• Two-point calibration: Calibration with two standard solutions.
• Multi-point calibration: Calibration with more than two standard solutions.
4. Overview and Installation
4.1 Overview
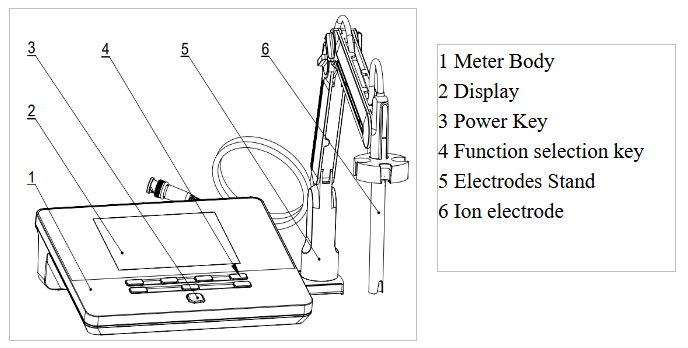
Figure 1
Overview-Front View
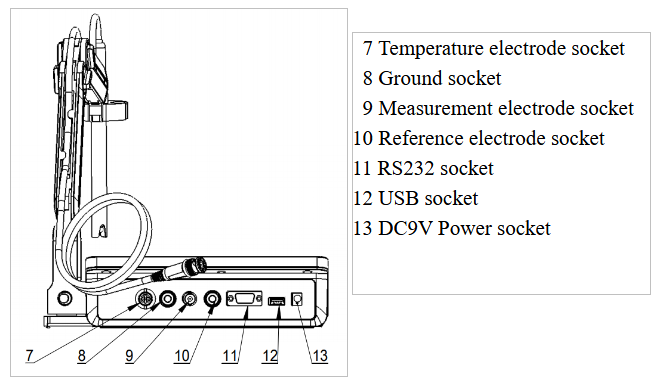
Figure 2
Overview-Back View
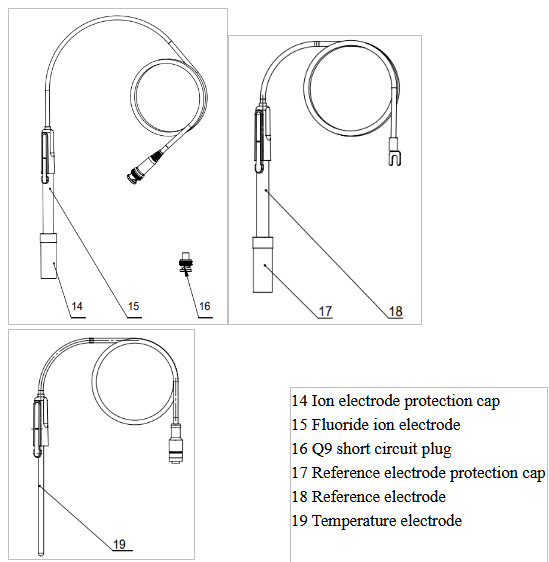
Figure 3
Electrodes and connectors
Connector Specifications
| Connector | Electrode type |
| BNC(Q9) | pH, ion selective electrode |
| Banana | Reference electrode |
Table 3
4.2 Instrument Installation
4.2.1 Electrodes Stand Installation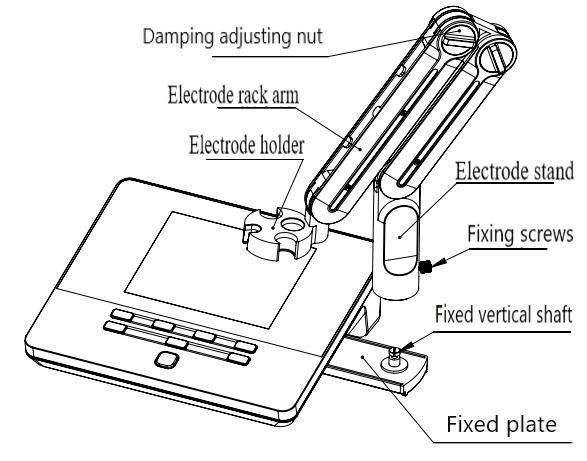
Figure 4
Electrode Stand Installation
Installation:
1) Pull out the electrode holder fixing plate on the right side of the instrument.
2) Insert the multifunctional electrode holder support (as Figure 4-4) into the vertical shaft of the multifunctional electrode holder drawer.
3) Tighten the set screw on the lower part of the electrode holder’s pole.
4.2.2 Electrodes Connection
Push the Fluoride ion electrode into the electrode holder. Find the measuring electrode socket on the back of the instrument, unplug the Q9 shorting circuit connector. Then, insert the fluoride ion electrode connector into the measuring electrode socket. When other electrodes are used as required, Insert the electrode connector to the corresponding socket on the back of the instrument in the same way as above.
5. Instrument Operation
5.1 Switch On/Off
After connecting the power adapter and installing the electrode stand and electrode, press to switch on the meter. The startup screen shows software version and other related information. After the self-test program, the screen turns to the homepage and the meter is ready to measure.
switch on the meter. The startup screen shows software version and other related information. After the self-test program, the screen turns to the homepage and the meter is ready to measure.The instrument uses touch keys as operation and control equipment, and is equipped with 8 keys in total. You can complete the corresponding operations by pressing the corresponding function keys.
Also press and hold
 the key for more than 3 seconds and release to shut down.
the key for more than 3 seconds and release to shut down.5.2 Screen Icons
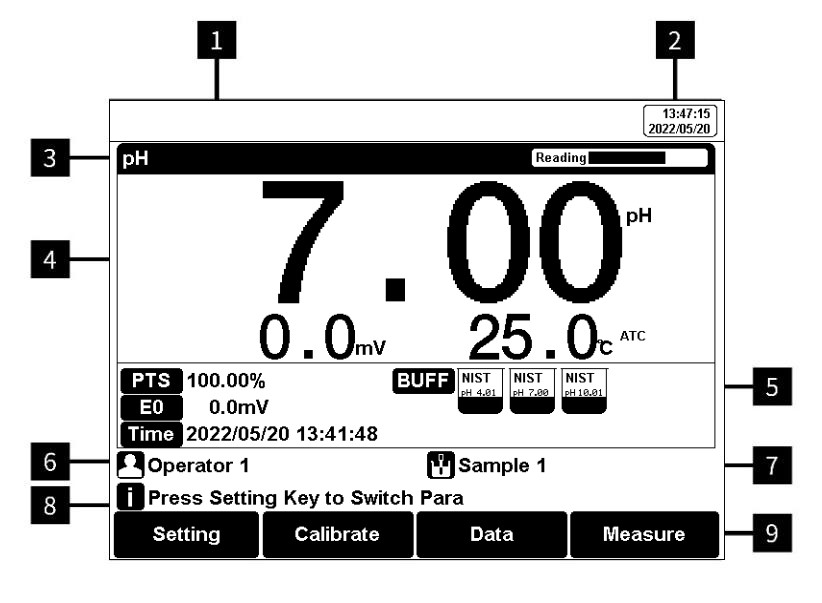
Figure 5
Screen icons explanation
1 Instrument model. 2 System time. 3 Measurement parameters &Reading status. 4 Main measurement box. 5 Calibration information. 6 User ID. 7 Sample ID. 8 Operation tips. 9 Soft function keys.
The instrument displays symbol identification that has the following functional implications:
Symbol Explanation
| No. | Symbol | Explanation |
| 1 |  | Reading status, display the measurement status of reading, stable, locked, each indicates that the processing, stable, and reading completed. |
| 2 |  | The percentage slope of the pH electrode calibration data. |
| 3 |  | The time of calibration |
| 4 |  | Measuring ions |
| 5 |  | Calibration point |
| 6 |  | Standard solution for pH calibration |
| 7 |  | Standard solution for ion calibration |
| 8 | 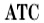 | Automatic temperature compensation |
| 9 | 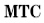 | Manual temperature compensation |
| 10 |  | User ID |
| 11 |  | Sample ID |
| 12 |  | Operation tips |
| 13 |  | The Standard buffer solution for calibration |
Table 4
5.3 Function Key
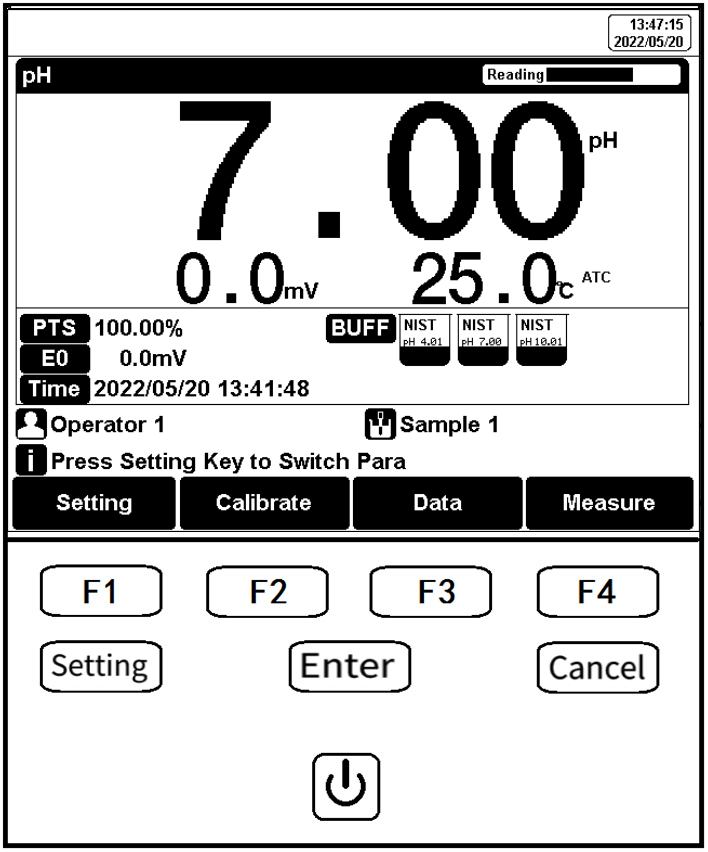
Figure 6
Function keys explanation
Key Function Explanation
| No. | Key | Explanation | Note |
| 1 |  | Power | Press to switch on/off |
| 2 |  | Setting | Set the parameters and settings |
| 3 |  | Cancel | Cancel the operation |
| 4 |  | Enter | Confirm the option |
| 5 |  | F1 | Function key, Corresponds to the function options on the screen |
| 6 |  | F2 | Function key, Corresponds to the function options on the screen |
| 7 |  | F3 | Function key, Corresponds to the function options on the screen |
| 8 |  | F4 | Soft function keys, corresponding to the functions on the screen |
Table 5
5.4 Instrument settings
In the measuring, users can set the instrument parameters by pressing "Setting" to set the measuring parameters.5.4.1 Tutorial setting
For the first use, please follow the guide to settings the measurement parameters. After all the settings, press the "Enter" to return to the previous page.
5.4.2 Measurement parameter settings
It could select one measurement parameter from pH, pX/ISE and Ion Conc.
5.4.3 Reading Mode Settings
The meter provides three reading modes, including continuous reading, auto reading, and timed reading.
• Continuous reading: The instrument displays real-time measurement results. User can end the measurement at any time and save the last result.
• Auto-reading: The measurement reached the balance, and the meter locked the reading result. The meter offers "Fast", "Medium", "Strict" and "Custom" four options for endpoint detection conditions.
• Time reading method: Timed Reading contains two kinds of timed reading methods: "Interval Measurement" and "Timed Measurement". "Interval Measurement" provide measurement results at interval time and "Timed Measurement" provide measurement result after a set time.
Reading Parameters Settings
| Stability Type | pH | pX/Ion concentration | |
| Fast | Stable time | 4s | 4s |
| Fluctuation | 1mV | 0.6mV | |
| Medium | Stable time | 6s | 8s |
| Fluctuation | 0.5mV | 0.2mV | |
| Strict | Stable time | 8s | 12s |
| Fluctuation | 0.1mV | 0.1mV | |
| Custom (Recommended value) | Stable time | 1 to 30s | 1 to 30s |
| Fluctuation | 0.1~1mV | 0.1~1mV | |
Table 6
5.4.4 pH parameter setting
5.4.4.1 pH standard solution group management
The meter provides various Standards Group including GB, DIN, NIST and USA. And allows the user to prepare the customized Standard groups.
Standard Solution Groups
| Groups | Contents |
| GB | 1.68pH, 3.56pH, 4.00pH, 6.86pH, 7.41pH, 9.18pH, 12.46pH |
| DIN | 1.68pH, 2.00pH, 3.56pH, 3.78pH, 4.01pH, 6.87pH, 7.00pH, 7.42pH, 9.18pH, 10.01pH, 12.45pH |
| NIST | 1.68pH, 4.01pH, 6.86pH, 7.00pH, 7.42pH, 10.01pH, 12.47pH |
| USA | 1.68pH, 4.01pH, 7.00pH, 10.01pH |
Table 7
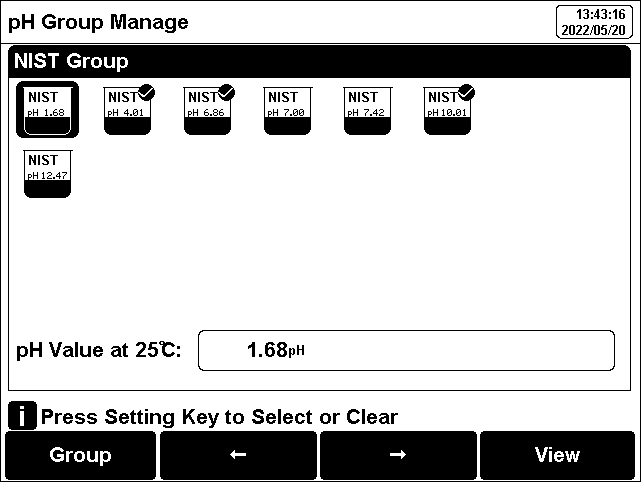
Figure 7
Selection of standard groups and standard solution
Usually we use the pH value corresponding to 25.0°C to mark the pH standard buffer solution, such as NIST 7.00pH standard solution, which means the standard pH buffer solution 7.00pH, and it is 7.00pH at 25.0°C.
After selecting the standard solution group, we need to select the standard buffer solution used for calibration from the standard solution group. The instrument supports up to 5-point calibration, that is, up to 5 standard solutions can be selected. Since the pH values of multiple standard buffer solutions in the standard solution group may be very close, to ensure that the instrument can correctly identify the standard buffer solution, will limit the selection of standard solutions with neighboring pH values. The check mark indicates the currently used standard solution group and the corresponding standard solution.
 TIPS:
TIPS:If the selected standard solution group is different from the pH standard buffer solution used, it will lead to wrong calibration results.
5.4.4.2 Manual standard solution identification
In some special cases, it is necessary to use some non-standard pH buffer solutions, or use two very close pH standard buffer solutions for electrode calibration. In this case, the manual standard solution identification function can be used. When set to "manual identification", the pH value of the current standard solution can be input during and used for electrode calibration.
5.4.4.3 Resolution settings
The pH measurement resolution of the instrument is adjustable.
pH resolution: 0.01pH and 0.1pH.
mV resolution: 0.1 mV and 1 mV.
5.4.5 pX/ISE parameter settings
5.4.5.1 Ion mode selection
Ion mode corresponds to pX, ion concentration measurement. The instrument supports conventional ion modes and user-defined ion modes. The instrument provides a variety of commonly used ion modes such as: F-, Cl-, Br-, I-, NO3-, BF4-, NH4+, K+, Na+, Ca2+, Cu2+, Pb2+, Ag+ and etc., which are convenient for use. Users can directly measure the concentration of the corresponding ions as long as they are equipped with the corresponding ion selective electrode and reference electrode.
The instrument allows the creation of custom ions, up to 6 types. Press the "Create" key, enter the ion name (maximum 8 characters), then enter the molecular weight (molar mass), and select the ionic valency.
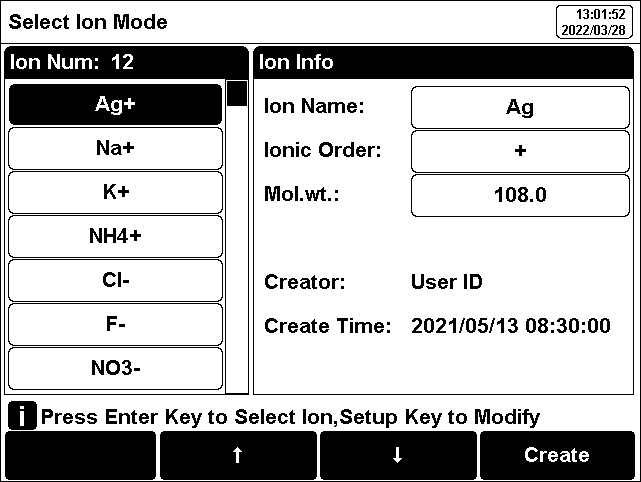
Figure 8
Manage and select ion modes
5.4.5.2 Select measurement mode
BMET-503 pH/on meter supports direct reading concentration method to measure ion concentration, also known as the standard curve method, is the most commonly used method for measuring ion concentration. This method establishes a linear relationship between the ion concentration and the electrode potential according to the Nernst formula:
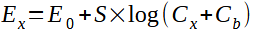
In the formula:
Ex~ Equilibrium potential of the sample to be tested, in mV,
E0~ Zero potential value, in mV,
S ~ Electrode slope (%),
Cx~ The concentration value of the sample to be tested, in mol/L,
Cb~ Blank concentration value, in mol/L.
By calibrating with a known concentration standard solution, the slope and zero potential value can be obtained, and a calibration curve can be established. When measuring an unknown sample, the corresponding concentration value can be read on the calibration curve through the measured potential value. The direct reading method has a fast measurement speed and is suitable for a large number of samples' fast measurement.
The result unit, blank concentration value, pX resolution and mV resolution can be set before measurement. Among them: pX measurement supports two resolutions, 0.01pX and 0.1pX, mV supports 0.1 mV and 1 mV.
5.4.6 Temperature Parameter Settings
The temperature unit of the meter is selectable in °C and °F.
Temperature compensation mode: ATC and MTC.
ATC means automatic compensation.
MTC means manual compensation. It allows user to input the temperature.
5.4.7 Data Management Settings
5.4.7.1 Sample ID setting
The instrument supports three setting methods of Sample ID: Auto sample ID in number order, Auto sample ID in time order, and manual sample ID.
• ID in number order: The sample ID No. is increasing with series number, allow to set ID digits (3 to 5 digits) and initial sample ID.
• ID in time order: The sample ID No. is increasing with sample measuring time. Format, Year/Y, Month/M, Day/D, Hour/H, Minutes/M, Second/S
• Manual sample ID: Manually set the sample ID No when saving or printing data.
5.4.7.2 Autosave setting
When this function is enabled, the meter saves the results when the reading is stable in the auto-reading and interval timed reading mode.
5.4.7.3 Overwrite setting
The meter provides 500 sets of measurement results storage space. When this function is enabled, the results data that exceeds capacity will overwrite the old results data.
5.4.8 Output option
Output the measurement results by selecting the data format.
The data formats are GLP, STD, and Custom.
5.4.9 User ID Settings
Allow to set the user ID.
5.4.10 System Parameter Settings
5.4.10.1 System time settings
Settings of system time and date.
5.4.10.2 Buzzer settings
Users can set the key sound by this setting.
5.4.10.3 Backlight settings
Users can adjust the screen brightness by this setting.
5.4.10.4 Automatic shutdown settings
The meter provides auto shutdown function. When the meter is not using and set the auto shutdown, the meter switches off automatically. There are six options: off, 1min, 2min, 3min, 5min, 10min, 15min, 20min, 30min, and 60 min.
5.4.10.5 Reset settings
The meter supports "Reset Measure Parameter" and "Reset All".
"Reset Measure Parameter" will reset measurement parameters to factory default and reset the calibration data to factory default calibration data.
"Reset All" will reset user ID and measurement parameters to factory default, and delete all results.
5.4.10.6 Software version
It shows software version information.
5.5 pH Measurement
5.5.1 Calibration preparationThe electrode slope and zero potential of pH electrodes drift slightly over time. To accurately measure pH, it is recommended to calibrate the pH electrode before use, the instrument supports 1-5 points calibration.
One point calibration is a calibration process with a single standard solution, commonly applied in a quick test. The calibration slope is 100% in here.
Two-point calibration is to use two pH standard buffer solutions to calibrate the electrode, and construct a linear calibration curve through two points. Two-point calibration is the most commonly used calibration method, and it is usually recommended that the pH value of the solution to be measured lies between the two standard buffer solutions. Two-point calibration can improve pH measurement accuracy.
Multi-point calibration is a calibration process with more than one standard solution. It is recommended to calibrate between two standard buffer solutions at the pH of the solution to be tested. Multi-point calibration covers a wider measurement range for accurate pH measurement. Before starting calibration, please prepare one or more pH standard buffer solutions.
5.5.2 pH electrode calibration
The calibration process is as follows:
1. Setting.
1) Set the parameters (e.g. pH).
2) Select NIST standard solution group, and check pH 4.01, pH 7.00 and pH 10.01 three standard solutions.
3) Set the Auto Mode recognition.
2. Press the F2"Calibrate"-"pH Calibration".
3. Put the cleaned electrode into pH 4.01 standard solution.
4. Wait for the instrument to display "Auto Mode Matched", or the instrument reading is stable, press the F4 "Start".
5. If only 1-point calibration is required, after 1-point calibration is completed, press the "Enter" key to complete the calibration.
6. If multi-point calibration is required, please replace the pH7.01 and pH10.01 standard buffer solutions. After cleaning the electrode, put the electrode into the standard solution. After the instrument recognizes it successfully, the instrument reads stably, press the F4"Next Point" to complete the calibration.
7. After completing the calibration, press the "Enter" key to complete the calibration, save the calibration results and end the calibration, directly enter the start interface. If the checked standard solution group is 5, automatically end the calibration after five points of calibration.
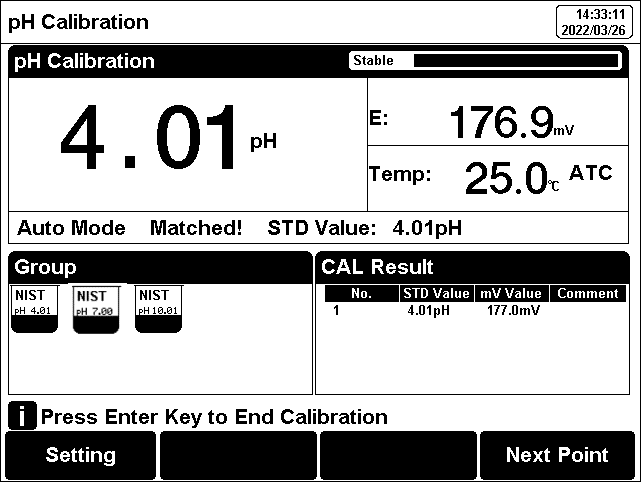
Figure 9
pH electrode calibration
5.5.3 pH measurement
The measurement process is as follows:
1. Setting.
1) Set the parameters (e.g. pH).
2) Set the reading mode (e.g., continuous reading, auto-reading, or timed format).
2. Put the electrode into test solution under test.
3. In the idle status, press F4 "Measure" to enter into measurement status.
4. When the reading is stable, press "Enter" to read the results.
5. Press the "Save" to save the measurement results.
6. Press the "Output" to print the measurement result when connect to the printer.
7. Between measurements, stored pH electrode in distilled or deionized water.
8. After measurement, rinse the pH electrode with deionized water thoroughly.
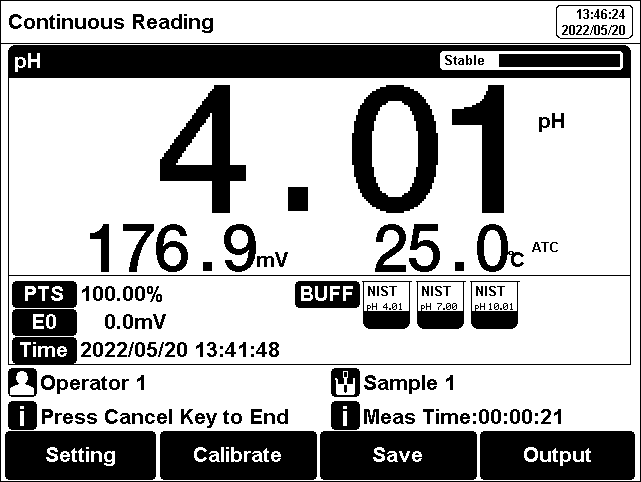
Figure 10
pH measurement information
 TIPS:
TIPS:➤ The measurement end of the electrode should well be immersed into the sample solution.
➤ For high accuracy measurement, make sure the measurement is carried out at the lab with constant temperature and pressure.
➤ If the two temperatures are different, it is recommended to use a pH combination electrode with temperature compensation or use a separate temperature electrode for automatic temperature compensation. Or use a thermometer to measure the temperature of the current solution, and manually set the temperature for manual compensation.
5.6 Ion measurement
5.6.1 PreparationThe slope and zero potential value of the ion electrode will change with time, so the ion electrode needs to be calibrated before use, and the instrument supports up to 5-point calibration. For the specific use of the electrode, please refer to the electrode manual.
5.6.1.1 Ion-selective electrode
The ion-selective electrode is based on the ion-selective membrane, which can be divided into single crystal membrane, salt membrane, glass membrane and PVC ion-selective membrane. Ion-selective electrodes usually have single electrodes and composite electrodes. Single electrode can be used with different reference electrodes, and has better measurement performance for some low-concentration ions. Composite electrodes are more convenient and
simpler in the operation. The meter requires the composite electrode. You could be flexible to choose according to the requirements.
5.6.1.2 Ionic strength adjustment buffer
The use of ion electrodes to measure ion concentration requires the addition of ionic strength adjustment buffer.
The ionic strength of a solution has an important influence on the measurement of ion concentration. On the one hand, the ion-selective electrode directly measures the activity of the ion, α = γc. Wherein, α is the activity of the ion, γ is the activity coefficient of the ion, and c is the ion concentration. Usually, the activity coefficient γ is affected by the ionic strength in the solution. By adding ionic strength adjustment buffer to the standard solution and the test solution, the measured solution has a similar ionic strength to the standard solution, thereby having a similar activity coefficient γ. On the other hand, is a solution with low ionic strength, the potential of the reference electrode will show instability. The addition of the ionic strength adjustment buffer can help stabilize the reference electrode.
Various ion measurement needs various ionic strength adjustment buffer. Common ionic strength adjustment buffers are recommended in the following table
Recommended ionic strength adjustment buffer
| Ion category | Ionic strength adjustment buffer |
| Na + | 0.2 mol/L diisopropylamine |
| F - | 0.1 mol/L NaCl or TISAB |
| Cl - | 0.1 mol/L KNO 3 |
| Br - | 0.1 mol/L KNO 3 |
| I - | 0.1 mol/L KNO 3 |
| Ag + | 0.1 mol/L NaNO 3 |
| Cu 2+ | 0.1 mol/L NaNO 3 |
| Pb 2+ | 0.1 mol/L KNO 3 |
| S 2- | 0.1 mol/L KNO 3 |
| K + | 0.05 mol/L MgAc 2 |
| Ca 2+ | 0.1 mol/L KCl |
| NO 3 - | 0.1 mol/L NaH 2 PO 4 |
| BF 4 - | 0.1 mol/L Na 2 SO 4 |
| ClO 4 - | 0.1 mol/L NaCl |
Table 8
* The final concentration of the ionic strength adjustment buffer in the standard or sample.
5.6.1.3 Standard solutions preparation
The best way to prepare standards is to use serial dilutions. Sequential dilution refers to diluting an initially prepared standard using a volumetric flask to obtain a second standard. Dilute the second standard to prepare a third standard. And so on until the required standard solution is obtained.
In general, the concentration between two adjacent levels is a 10-fold relationship.
5.6.1.4 Activation of the ISE electrodes
When the electrode is used for the first time or has not been used for a long time, an activation is recommended. The electrode has better measurement performance after activation.
Ion electrode activation solution and activation time recommendation
| Ion category | Activation solution | Activation time |
| Na + | 10 -3 mol/L NaCl | 2h |
| F - | 10 -3 mol/L NaF | 2h |
| Cl - | 10 -3 mol/L KCl | 2h |
| Br - | 10 -3 mol/L NaBr | 2h |
| I - | 10 -3 mol/L NaI | 2h |
| Ag + | 10 -3 mol/L AgNO 3 | 2h |
| Cu 2+ | 10 -3 mol/L Cu(NO 3 ) 2 | 2h |
| Pb 2+ | 10 -3 mol/L Pb(NO 3 ) 2 | 2h |
| S 2- | 10 -3 mol/L AgNO 3 | 2h |
| K + | 10 -3 mol/L KCl | 2h |
| Ca 2+ | 10 -3 mol/L CaCl 2 | 2h |
| NO 3 - | 10 -3 mol/L NaNO 3 | 2h |
| BF 4 - | 10 -3 mol/L NaBF 4 | 2h |
| ClO 4 - | 10 -3 mol/L NaClO 4 | 2h |
Table 9
 TIPS:
TIPS:The activation time may variously be based on various activation solutions. See the ion-selective electrode manual for specifications.
5.6.1.5 Stirrer setting
The flow state of the solution influences the electrode potential of the ion-selective electrode. To improve the stability and repeatability of the measurement, it is recommended to use a stirrer to keep the flow rate of the solution stable during calibration and measurement.
5.6.2 pX/ISE Calibration
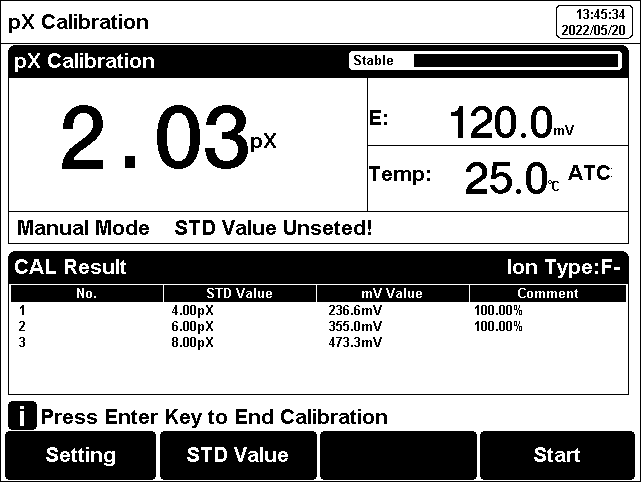
Figure 11
pX/ISE calibration information
The pX/ISE calibration process is as follows:
1. Add an appropriate amount of standard solution (usually 100 ml) to the beaker, then add ionic strength adjustment buffer. Adjust the stirring speed of the solution for measurement.
2. Press the F2 "Calibrate"-"pX Calibration".
3. Put the cleaned electrode into standard solution.
4. Press the F2 "STD Value" to input the standard value of the standard solution.
5. Wait for the reading is stable, press the F4 "Start".
6. If only 1-point calibration is required, after 1-point calibration is completed, press the "Measure" to complete the calibration.
7. If choosing multi-points calibration (up to 5), press "Next Point" to repeat the operation.
8. If the checked standard solution group is 5, automatically end the calibration after five points of calibration.
 TIPS:
TIPS:➤ Please recalibrate for an unexpected measurement result.
➤ A room temperature test solution is recommended.
➤ It is recommended to calibrate from low concentration to high concentration standards.
5.6.3 pX/ISE Measurement
Select the ion measurement methods by user needs. In general, when direct-reading method is using for ion concentration measurement and pX measurement.
The measurement process is as follows:
1. Setting.
1) Set the parameters (e.g., pX).
2) Set the ion mode (e.g. F-).
3) Set the direct Reading as concentration meas mode.
4) Set the concentration unit (e.g. ppm).
5) Set the reading mode (e.g., continuous reading, auto-reading, or timed format).
2. Add an appropriate amount of standard solution (usually 100 ml) to the beaker, then add ionic strength adjustment buffer. Adjust the stirring speed of the solution for measurement.
3. In the idle status, press F4"Measure" to enter into measurement status.
4. When the reading is stable, read the results.
5. Press the "Save" to save the measurement results.
6. Press the "Output" to print the measurement result when connect to the printer.
7. Between measurements, stored ISE electrode in distilled or deionized water.
8. After measurement, rinse the ISE electrode with deionized water thoroughly.
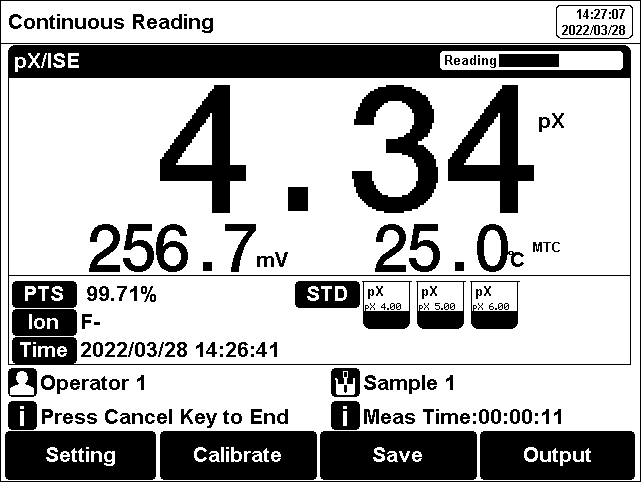
Figure 12
PX/ISE measurement information
 TIPS:
TIPS:Different ISE probes have different potential values in a blank solution. If the blank potential is away from the reference value, the user can do an activation to improve the performance of electrodes. If the electrode still does not meet the requirements, a new electrode is quite considerable.
5.7 Data Management
Press "Data" to view the detail of results.The meter stores the measurement results independently according to the measured parameters. The meter provides data Storage 500 sets for each parameter (pH/mV/Temp).
The user can press "Delete" into the delete menu. It allows users to select the parameter data or all data to delete.
The user can view the data filter by parameter, locate No. or stored date. By the filter setting, press "Start Search" to look up the data. The filter data shows in a graph. Press "←" and"→" to choose data. User can choose one and press "Enter" key to see the detail result. Users can press "Delete" to delete the current result. Press "Output" to select the output data. The format supports output the current result, output matched result and output all result.
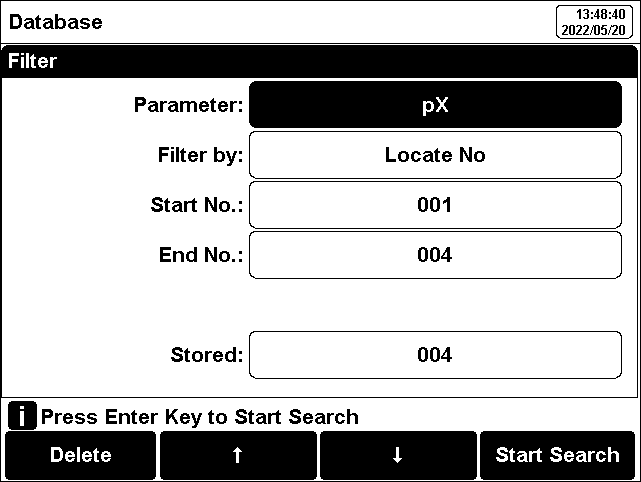
Figure 13
Results setting view
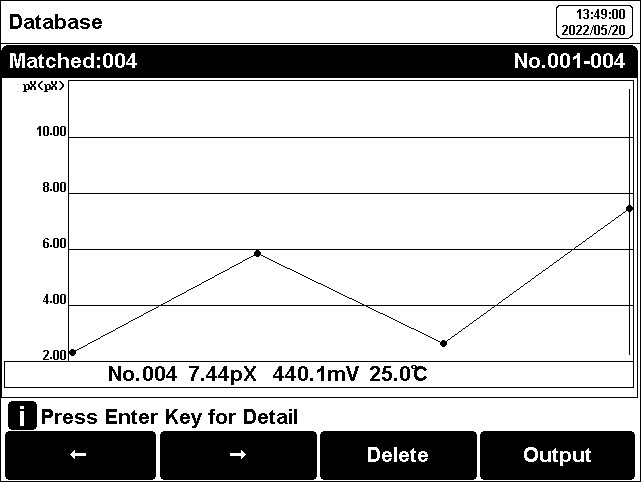
Figure 14
Review stored results by graph
 TIPS:
TIPS:➤ In order to ensure the correct use of the instrument and avoid burning the instrument and causing unnecessary losses to you, please turn off the power of the instrument and printer before connecting the printer.
➤ The communication baud rate of the meter is always 9600bps, the default setting is 8 data bits, one start bit, one stop bit, no parity.
Output format is approximately as follows:
****************************************
Report Title
----------------------------------------
Measure Time:2021/01/19 12:27:28
Operator: Operator 1
Model: BMET-503 pH/Ion Meter
Serial Number:
SW Version: Ver 1.00
----------------------------------------
............................MATCHED INFO
Stored Num: 28
Matched Num: 1
Stored No.: 15
..............................CALIB INFO
Calib Operator: REX Team
Calib Time: 2020/05/13 08:30:00
Calib Num: 3
............................CALIB RESULT
STD 1: 4.00pH 177.3mV 25.0c
STD 2: 6.86pH 8.0mV 25.0c
STD 3: 9.18pH -129.1mV 25.0c
pH Slope 1: 100.00%
pH E0 1: 0.0mV
pH Slope 2: 100.00%
pH E0 2: 0.0mV
..............................BRIEF INFO
Reading Mode: Timed Reading
Stable Type: Medium
Temp Comp Type: ATC
.............................SAMPLE INFO
Sample ID: Sample 1
..................................RESULT
Result: 7.00pH
Signal Value: -0.0mV
Temp Value: 25.0c
----------------------------------------
----------------------------------------
6. Maintenance/Troubleshooting
6.1 Meter Maintenance
The correct use and maintenance of the instrument can ensure the accurate and reliable performance of the instrument. Additionally, exposure to chemicals or harsh use environments can affect performance.The pH/pX electrode socket has a protective plug, when the meter is not in use, please insert the protective plug into the pH/pX socket.
• If the meter is not used for a long time, please disconnect the power supply.
• The electrode socket of the instrument must be kept clean and dry, and should not be in contact with acid, alkali, and salt solutions.
• Keep the meter and accessories clean and away from acids, alkalis, and any corrosive solutions/gases.
• Users can clean the meter surface with clean waters and detergent.
• When the meter is transported, please follow the instructions:
• please remove all connected cables.
• Please remove the electrode holder.
• Please use original packaging in the long-distance transport to avoid damage.
6.2 Electrodes Maintenance
For more detailed information, please refer to the electrode instruction manual.6.3 Troubleshooting
| Phenomenon | Probable reasons | Solutions |
| 1. No Display | Not powered on. Damage to the meter. | Connect the adapter and press the power key to turn it on. Replace or repair as required. |
| 2. Incorrect mV measurement is | 1. The electrode is out of service life 2. The electrode plug is in poor contact | 1. Replace the electrodes 2. Connect the protection plug, if the potential is not 0mV, please contact the after-sales service. |
| 3. Incorrect pH measurement | 1. Refer to as 2.2 2. Refer to as 2.2 3. The electrodes are not calibrated or are calibrated incorrectly | 1. Refer to as 2.2 2. Refer to as 2.2 3. Recalibrate the electrode or replace the standard solution |
| 4. Incorrect pX/ISE measurement | 1. Refer to as 2.2 2. Refer to as 2.2 3. The electrodes are not calibrated or are calibrated incorrectly 4. Incorrect ISE probe | 1. Refer to as 2.2 2. Refer to as 2.2 3. Recalibrate the electrode or replace the standard solution 4. Buy correct ISE probe. Add ionic strength adjustment buffer. |
Table 10
If the meter still does not work, please contact your local dealer for further assistance.
7. Technical Supports
AccessoriesPlease refer to the accessories table for purchasing recommendations.
Meter accessories
| Name | Description |
| E-301-QC 3 in 1 pH composite electrode | pH Measurement Probe |
| PF-2-01 Fluoride ion electrode | Measure the fluoride ion content |
| 232-01 Reference electrode | Use with ion electrode |
| T-818-Q Temperature electrode | Measure temperature value |
| REX-5 Electrode stand | Place electrodes during measurement |
| pH 4.01/7.00/10.01 standard sachets | To prepare the standard |
Table 11
8. Appendixes
Appendix 1pH-Temperature Relationship Table of pH Standard Solutions
| Temperature(°C) | 1.68 | 4.01 | 7.00 | 10.01 |
| 5 | 1.67 | 4.00 | 7.09 | 10.25 |
| 10 | 1.67 | 4.00 | 7.06 | 10.18 |
| 15 | 1.67 | 4.00 | 7.04 | 10.12 |
| 20 | 1.68 | 4.00 | 7.02 | 10.06 |
| 25 | 1.68 | 4.01 | 7.00 | 10.01 |
| 30 | 1.68 | 4.01 | 6.99 | 9.97 |
| 35 | 1.69 | 4.02 | 6.98 | 9.93 |
| 40 | 1.69 | 4.03 | 6.97 | 9.89 |
| 45 | 1.7 | 4.04 | 6.97 | 9.86 |
| 50 | 1.71 | 4.06 | 6.97 | 9.83 |




