Home Clinical Microbial Diagnosis
Clinical Microbial Diagnosis
 Identification And Antibiotic Susceptibility Testing
Identification And Antibiotic Susceptibility Testing
 Microbial ID/AST System BCMD-101
Microbial ID/AST System BCMD-101
 Clinical Microbial Diagnosis
Clinical Microbial Diagnosis
 Identification And Antibiotic Susceptibility Testing
Identification And Antibiotic Susceptibility Testing
 Microbial ID/AST System BCMD-101
Microbial ID/AST System BCMD-101
Microbial ID/AST System BCMD-101
- Sea, Air, Door to Door Shipping
- 1 Year Warranty
- US & European Standards
The device offers quick and precise species-level identification of clinically significant pathogenic bacteria and yeasts based on the most recent microbial categorization system.
- Colorimetric method for microorganism identification and turbidity method for antibiotic susceptibility testing (AST)
- Powerful QC function can meet the quality control requirement of any size lab.
- Connect with users� LIS and transfer data to WHONET
Specification
Features
Applications
| Detection Method | Photoelectric micro-well by micro-well detection with 8 road detection unit |
| ID/AST results | Provided in 18-24 hours, as little as 4 to 6 hours |
- Colorimetric method for microorganism identification and turbidity method for antibiotic susceptibility testing (AST)
- Powerful QC function can meet the quality control requirement of any size lab.
- Connect with users� LIS and transfer data to WHONET
Microbial Diagnosis, Epidemiological Investigation, Drug Resistance Trend Analysis and Hospital Infection Management.
Operating Manual for BCMD-101
1. Product overview
1.2 antibiotic susceptibility identification process
2. System composition
2.1 Specimen processing
2.2 Preliminary identification of bacteria
3. Installation of the instrument
4. Identification and antibiotic susceptibility interpretation procedures
4.1 Instructions for operation of the instrument
4.2 Safety protection
4.3 Starting up
4.4 Run the identification program
4.5 Add specimen information
4.6 Bacteria identification and antibiotic susceptibility analysis
5. Query and statistics
5.1 Query and printing of identification report
5.2 Query of nosocomial infection surveillance reports
5.3 Bacterial detection rate statistics
5.4 Antibiotic sensitivity statistics
5.5 Bacteria-antibiotic sensitivity statistics
5.6 Analysis of bacterial detection trends in different quarter
5.7 Statistics of enzyme-producing strains
5.8 Department submitted quantity statistics
5.9 Statistics of test specimen count
5.10 Statistics of tested bacterial count
5.11 Other statistics
6. System settings
6.1 Purpose of system setting
6.2 Content items of system settings
6.3 Setting operation of the system
7. Tools
7.1 Antibiotic data
7.2 antibitoic susceptibility test description
7.3 LIS comparison table
7.4 WHONET
7.5 Backup/restore
8. Help
8.1 About
9. Use and maintenance of the instrument
9.1 Working conditions
9.2 Scope of application
9.3 Contraindications
9.4 Cautions, warnings and suggestive instructions
9.5 Maintenance methods
9.6 Warranty period
9.7 Discontinuation of the equipment due to maintenance, transportation or handling
10. Electromagnetic compatibility declaration
10.1 Sample cables
1. Product overview
1. The system can be used for in vitro bacterial identification and antibiotic sensitivity test.2. The common clinical microorganisms that can be identified: enterobacteriaceae, non-fermentative bacteria, staphylococcus, streptococcus, yeast-like fungi, etc.
3. It can be used for the semi-quantitative analysis of antimicrobial susceptibility to enterobacteriaceae, non-fermentative bacteria, staphylococcus, streptococcus, yeast-like fungi, and so on.
4. It has the function of automatically identifying and analyzing the abnormal information during the test, and giving an alert; and, screening out the MRS, MRSA, ESBLS, CRE, HLAR and other drug resistance mechanisms.
5. The supporting kits include: the in vitro diagnostic kit, the microbial identification biochemical kit, and the yeast-like fungus antibitoic susceptibility kit provided with the BCMD-101 together.
6. The detection system reads and analyzes the data through photoelectric detection.
7. Information processing: query of bacterial identification and antibitoic susceptibility analysis information, and the statistical analysis on bacteria and antibiotics are available.
8. Nosocomial infection surveillance: the surveillance results of nosocomial infection can be recorded, queried, analyzed and reported.
9. An alert will be given when there is any error or abnormality.
1.2 antibiotic susceptibility identification process
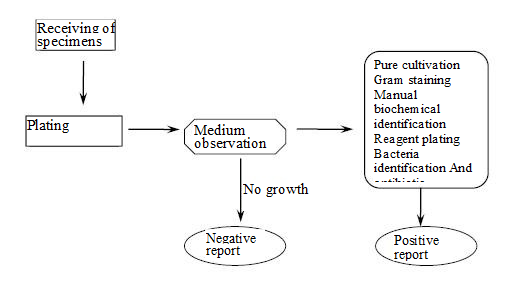
(Fig.1-1 antibiotic susceptibility identification process)
First of all, the pure cultured specimen strain should be prepared into bacterial suspension, which should be added into the kit according to the specified requirements. Then, the kit should be placed in an incubator at 35℃ for incubation of 18-24 hours. Then, the detection system automatically detects the kit according to the instructions, and records the spectral and absorbance values of each hole. The analysis system automatically processes the information received to give the bacterial species and antibitoic susceptibility test results. Finally, you can output the report via the printer. This is shown in Fig. 1-1.
2. System composition
| Composition and structure | XK-Ⅱ | XK |
| Composition | Detector mainframe, system analysis | Detector mainframe, computer, printer-for |
| software. | option, system analysis software. | |
| Structure | The touch all-in-one machine is built into the mainframe and can be controlled by touch. | The computer monitor is placed outside mainframe of the instrument and cannot be controlled by touch. |
This is shown in Fig. 2-1.
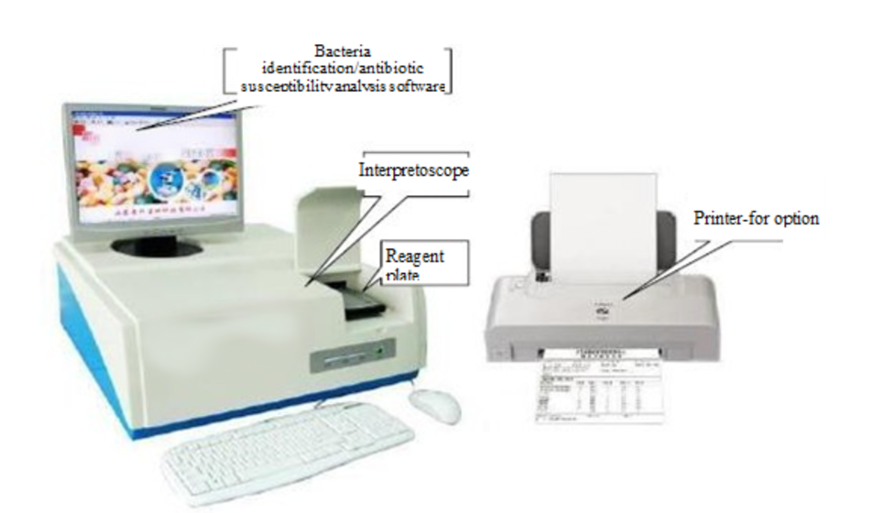 (Fig.2-1 Composition of BCMD-10)This is shown in Fig. 2-2.
(Fig.2-1 Composition of BCMD-10)This is shown in Fig. 2-2. 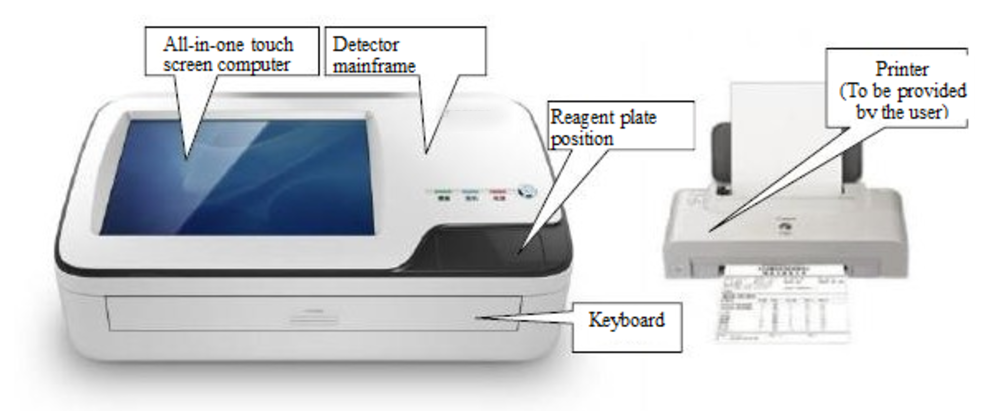
(Fig.2-2 Composition of XK-Ⅱ BCMD-101)
2 Operation procedures
2.1 Specimen processing
2.1.1 Specimen collection: Different methods and instruments should be used according to different specimens/parts/objects. The specimens should be taken according to the aseptic operation procedures, in order to avoid them from being contaminated.2.1.2 Specimen cultivation: Different media and cultivation methods should be used for different specimens, and the suspicious colonies should be selected and purified according to the growth of bacteria.
Note: The unpurified strains may be contaminated by miscellaneous bacteria, which may affect the accuracy of identification results.
2.2 Preliminary identification of bacteria
In order to select an appropriate kit, preliminary identification classification must be carried out.Note: The incorrect preliminary identification will directly lead to completely wrong results.
1.Staining and microscopy
The bacteria should be stained strictly according to the gram staining standards, and morphology of the bacteria should be determined by microscopy.
2.3 Manual test
In order to select an appropriate kit, the oxidase, catalase and OF test should be carried out first. For details, please see Table 3.1.
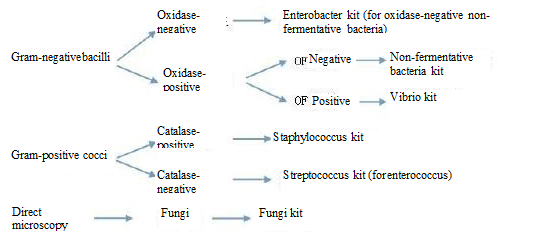
Table 3.1
3. Sample addition and cultivation operation of the kit
For details of the operation methods, see the operation manual of each kit.
4. Interpretation and analysis
1.First turn on the "I" and "O" power switch on the back of the instrument, then start the computer, and run the software of "BCMD-101".
2. Start system testing and analysis (please follow the identification procedures).
3. Print the report.
3. Installation of the instrument
All the installation and debugging of the instrument shall be conducted by the engineer designated by the manufacturer on the spot, and the user shall conduct the instrument acceptance.1. Configuration and related instructions
1. Unpacking inspection:
1. The instrument packing case should be opened, and the instrument and accessories shall be carefully taken out from the case. The items contained in the packing case are shown in the attached packing list. After unpacking, the instrument and all accessories should be carefully checked to ensure that the items are complete. If any part is found to be missed, please inform the manufacturer or dealer immediately.
2. Packing list of the mainframe:
Table 4-1 Packing List of the mainframe
| Specification model | XK | XK-Ⅱ |
| Instrument mainframe (including power cord) | √ | √ |
| Monitor (including video cable and power cord) | √ | All-in-one touch screen computer (built-in) |
| Keyboard and mouse set | √ | √ |
| Printer (set) | For option | -- |
| Certificate | √ | √ |
| Instruction | √ | √ |
| Warranty card | √ | √ |
3.1.1 Weight of main components: Instrument mainframe 30kg
3.1.2 . Environment
3.1.3Working environment
The instrument shall be installed and operated in the following working environment:
-Ambient temperature: 5℃ - 40℃;
-Relative humidity: ≤70%;
-Power supply: AC220V±10%, 50Hz±10%;
-Input power: 400VA;
-The interference source of strong electromagnetic field should be far away, and the direct irradiation of strong light should be avoided;
-The working site shall be protected from dust and vibration; the air shall not contain any acid, salt and other corrosive gases;
-The grounding environment shall be good;
-The space with the wall should be no less than 20cm, in order to protect the power supply and data cable, and to facilitate the heat dissipation;
-There should be sufficient operation and maintenance space for operators, which should meet the requirements of relevant regulations.
-The instrument should not be placed in a location that prevents the staff from being able to switch power supply of the instrument on and off properly.
3.2.2 Transportation and storage environment
3.2.2.1
Because there is no liquid substance inside the instrument, the instrument will not produce any potential danger when it arrives at the use unit after normal transportation.
The instrument is a precision testing instrument which should be transported and stored with care to avoid damage. When being transported, the words and marks such as "Handle with Care", "Keep Upwards" and "Keep Dry" should be marked. Strong impact, rain and sun exposure must be prevented during transportation. During transportation, the responsible person shall transport and store according to the following environmental requirements:
-Ambient temperature range: -20℃~55℃;
-Relative humidity: ≤80%
-Atmospheric pressure: 50.0kPa ~ 106.0kPa;
-Strong impact, rain and sun exposure must be prevented;
-The instrument should be stored in the direction indicated by the "Keep Upwards" arrow marked on the instrument;
-It should be "handled with care".
3.2.2.2 Description of marks
 | "Handle with Care" mark. The instrument is a precision testing instrument and should be handled with care. |
 | "Up" identification |
 | “Keep Dry” mark. It should be kept dry so as not to affect the equipment performance |
 | “Do Not Roll” mark |
Table 4-2 Description of packaging marks
Power supply requirements
The rated voltage of the instrument is AC220V50Hz and the input power is 400VA. There should be a stabilized power supply between the power supply and the system, and the installation of the power cord should be safe and reliable. The capacity of the fuse is indicated on the box at the back. The specific model is F2AL250V. The user shall not replace it without authorization. If there is any abnormality, please contact Biolab or its dealer.
 Cautions : The instrument should be prepared prior to use in accordance with the procedures and requirements described in this chapter.
Cautions : The instrument should be prepared prior to use in accordance with the procedures and requirements described in this chapter.1. Installation environment
-It is intended for indoor use and shall be avoided from direct sunlight
-There should be no large amount of dust
-There should be no strong electromagnetic radiation
-The gro1. Before this step, please read this Manual carefully and operate according to the requirements.
2. Please connect the power cord to a power outlet which is correctly connected and properly grounded.
3. Disconnect the connected power cord or other wire before opening the device housing unless otherwise stated.
4. Do not plug or unplug any cable, or install or maintain the product during lightning.
5. Pay attention to keep the socket within the load capacity to prevent fire.
6. The instrument should be equipped with protective grounding.
7. The grounding wire of the three-core power cord must be effectively and directly grounded, and the grounding resistance should be less than 0.1 ohm, in order to avoid accidents.
4. Installation of BCMD-101
und should be flat and firm, and bearing capacity of the ground should meet the building requirements
The back of the instrument can be placed against a wall, but the distance from the wall should be greater than 20cm to ensure that air flow of the instrument fan will not be blocked, and to maintain the space and ventilation required for effective maintenance
- Room temperature 10℃ ~ 30℃, humidity RH≤ 80%
- The instrument shall be placed on a horizontal stable support or table with sufficient space and a load-bearing capacity of 50kg greater than the instrument weight
 Cautions : Please read this Manual carefully and operate according to the requirements.
Cautions : Please read this Manual carefully and operate according to the requirements.The equipment should be placed in a way that meets the above requirements, so as to ensure that the operator can easily and timely disconnect power supply of the equipment to ensure safety in case of an emergency endangering the safety.
2. Installation of mainframe
The XK shall be operated as follows:
Take out the instrument carefully and place it on a stable horizontal support or table. Open door of the instrument, take out the internal fasteners used for transportation in the instrument, and make sure that no irrelevant items is left in the instrument. Connect it to the computer with the data cable and connect the power cord.
The XK-Ⅱ shall be operated as follows:
Take out the instrument carefully and place it on a stable horizontal support or table. Open door of the instrument, take out the internal fasteners used for transportation in the instrument, make sure that no irrelevant items is left in the instrument, and connect the power cord.
Notice:
Do not plug and unplug any connection of the instrument when it is live, so as not to damage interfaces of the instrument.
3. Computer installation
The XK shall be operated as follows:
The computer should be installed according to the relevant operation instructions.
4.4.4Software setup
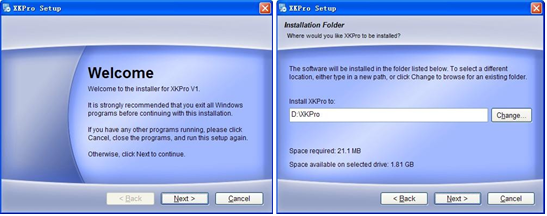
1. Software setup: Follow the prompts to complete the software installation step by step, as shown in Fig. 4-1.
Double click the setup icon to run the setup
(2) Determine the installation path

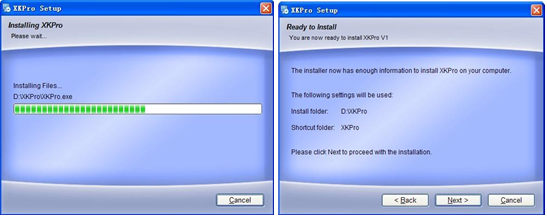
(Fig.4-1 Software setup)
(3) Installation is in progress
(4) Installation is completed
2. Software registration: For the first time to use the identification software, you need to register first, enter name of your company and press Enter to get the user ID; then you should provide the user ID to the manufacturer, and the manufacturer will return the registration code according to the user ID; finally, you need to enter the registration code to finish software registration.
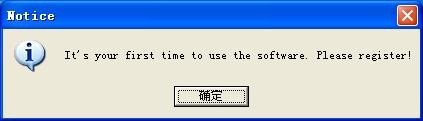
This is as shown in Fig.4.2 and Fig.4-3.
(Fig.4.2)
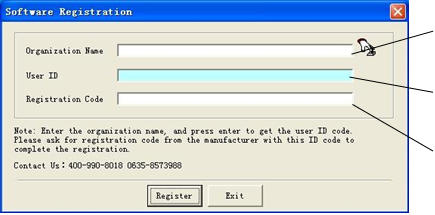
(Fig.4.3)


Printer installation: please follow the instructions for printer installation.
Start-up
Check whether the signal lines are well connected and whether the power cord is tightly connected before power-on. Make sure that the total rated current of the instrument devices is not higher than rated current of the power supply. Meanwhile, make sure that the total rated current of all electrical devices in the room is not higher than rated current of the fuse. Then switch the power on to start the instrument.
4. Identification and antibiotic susceptibility interpretation procedures
4.1 Instructions for operation of the instrument
4.1.1 Instructions for operation of the instrument Operate the power switch to start up: First, turn on the rocker main power switch with the sign of "I" and "O", and then turn on switch of information processing part (computer) of the instrument;4.1.2 The
 . on the instrument indicates the “biological hazard”; while, the
. on the instrument indicates the “biological hazard”; while, the 
indicates “Be careful, dangerous”; "IVD" is the identification of in vitro diagnostic medical device; the place marked with the "Watch Your Hands" is a moving part, and your hands should be kept far away from it during operation; both the using unit and the user should pay attention to and understand the danger indicated by this mark;
4.1.3 The location where the kit is going to be place has the potential risk of pinching hand. After placing the kit, your hands should be kept away from the location marked with "Watch Your Hands" before proceeding to the next step;
4.1.4 The dust inside and outside the instrument should be cleaned regularly (when the power is turned off);
4.1.5 Disinfection can be achieved by the indoor disinfection devices equipped in the space where the instrument is placed: for example, the ultraviolet sterilization lamp, ultraviolet sterilization car or ozone generator;
4.1.6 The instrument is going to be used with the special kits which are disposable. When the kits are used up, please contact our company or our authorized agents/dealers.
4.2 Safety protection
4.2.1. Because the specimens to be tested by the instrument have potential infectivity, the operator should wear safety gloves when conducting identification operation, and shall strictly follow the "Clinical Microbiology Laboratory Management System";4.2.2 The kits used for the instrument are disposable and cannot be reused. As the specimens to be tested are potentially infectious, they must be autoclaved and incinerated in accordance with the “Management Regulations on Medical Wastes”.
4.3 Starting up
First turn on the main power switch with 'I' and 'O' signs, and then press the computer switch to start the computer.4.4 Run the identification program
Select the set user name and enter the login password to log in the system.Note: The system user xk (the login password is xk) should be selected for the first login to set the user name and password.
4.5 Add specimen information
4.5.1 Click the "Application and identification" button, and enter and save the specimen information to be tested in the "Add specimen" field4.5.1.1 According to the testing needs, you can select the testing date by clicking the inverted triangle on the right of "Submission date". The system defaults to the current system date.
Note: The selected submission date cannot be later than the current system date.
4.5.1.2 After entering the [Out-patient/inpatient number], press the Enter key, and then the system will first search by out-patient/inpatient number. If application information of the out-patient/inpatient number is already available in the system, it will be automatically loaded, and the user can [add] it after confirming the information; Otherwise, the cursor will automatically move to the [Name] box and you should continue with the current entering operation.
4.5.1.3 After entering the [Name] and pressing Enter, the cursor will automatically move to the [Gender] box. You can select the gender by clicking the inverted triangle to the right of the box, or select the gender by clicking the "Up/down curser" key.
Similarly, you can press the information entry, Enter and "Up/down cursor" key to enter the [Age], [Age unit], [Ward bed number], [Specimen number], [Test specimen], [Test purpose], [Submitting department], [Submitting physician], and [Clinical diagnosis].
Note: For the [Test specimen], [Test purpose], [Submission department] and [Submission physician], you can set them in advance in the setting interface, or click the field following the option
 in this interface to add the required input items in an emergency.
in this interface to add the required input items in an emergency.Note: For the reports that are not set to link to the LIS system, the [Name], [Gender], [Test specimen], [Test purpose], and [Submission department] are required options. If any of them is blank, the next step cannot be proceeded to. For the reports that are set to link to the LIS system, the [Specimen number] and [Test specimen] are required options.
Note: When [Age] is not blank, the [Age unit] is required; when [Age] is blank, the [Age unit] is not required; when the [Age unit] is "Unknown", [Age] is blank and non-editable.
Note: [Clinical diagnosis] is optional. The existing description items can be directly selected. If no existing description items are available, you can add the required input items in an emergency by clicking the field following this option
 ; this information box can be left blank.
; this information box can be left blank.Note: the user can input the specimen number according to the numbering principle of our hospital.
4.5.1.4 When you click the [Add] after entering the information of a specimen submitted for testing, the system will be initialized into a new typing state and you can continue to type the information of a new specimen submitted for testing. The added specimens are shown in the "Specimens to be tested" field at the top.
4.5.2 Selection, emptying, modification and deletion of records of submitted specimens
4.5.2.1 By clicking the list of the "Specimens to be tested", you can select valid specimen information to be tested, and its details will be displayed synchronously in the corresponding information box.
4.5.2.2 When a test application record is selected, [Add] will become inoperable, and [Modify] and [Delete] will become operable, from which the selected record can be modified and deleted.
4.5.2.3 When the [Empty] is clicked: The "Add" key will be operable, the "Modify" key will be inoperable, and the message boxes will return to the initialized state.
4.6 Bacteria identification and antibiotic susceptibility analysis
4.6.1 Selection of specimens to be tested4.6.2. Click the list item of "Specimens to be tested" and select the specimen to be tested.
4.6.3 Selection of report date
4.6.3.1 You can select the report date by clicking the inverted triangle to the right of the "Report date". The system defaults to the current system date.
Note: The selected report date cannot be later than the current system date and cannot be earlier than the application date.
4.6.4 Interpretation of biochemical identification boxes
Note: The main form of the identification system is in an inoperable state both during the [Automatic identification] and [Manual identification].
Note: Before interpretation of the biochemical identification box, the relevant test description information matching the specimen can be selected according to needs, or it can be added instantly by clicking the key

followed after the tab.
4.6.4.1 Loading of the kit
Upper cover on interpretation chamber of the identification instrument should be opened, and the biochemical kit should be put into the card slot on interpretation chamber of the identification instrument.
Note: When placing the kit, please accurately place the kit into the small card slot near the inner part with the chamfered side facing inward.
Automatic identification: When selecting the "Bacteria category" in the bacteria identification field of the identification interface, and then clicking the [Automatic identification] button, the instrument will automatically test the kit that has been placed, and display the detailed test results.
Note: When you click the [Modify] button, you can modify state of a certain hole, and the [All negative] and [All positive] will be restored to be operable, and the [Modify] will be changed as [Identification].
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
Note: When you click the [Bacteria selection] button, you can enter the "Bacteria results" interface to directly select the bacterial results.
4.6.4.2 Manual identification: When you select the "Bacteria category" in the bacterial identification field of the identification interface, and then click the [Manual identification], you can enter the "Manual identification" interface, in which initial state of each biochemical item is negative. When you click the [Identification] button after selecting and confirming color state of each biochemical item in turn, the corresponding bacterial results will be displayed in the list automatically. At the same time, the button will be changed as [Modify].
Note: When you click the [Modify] button, you can modify state of a certain hole, and the [All negative] and [All positive] will be restored to be operable, and the [Modify] will be changed as [Identification].
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
Note: When you click the [Bacteria selection] button, you can enter the "Bacteria results" interface to directly select the bacterial results.
4.6.4.3 Identification modification: If you want to modify color state of a biochemical item after bacterial identification by automatic interpretation or manual interpretation, click the [Modify] button to make all holes modifiable, and then modify color state of one or more biochemical items. When color state of each biochemical item is confirmed after modification, click the [Identification] button to get the modified bacterial results.
Note: When you click the [Modify] button, you can modify state of a certain hole, and the [All negative] and [All positive] will be restored to be operable, and the [Modify] will be changed as [Identification].
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
Note: When you click the [Bacteria selection] button, you can enter the "Bacteria results" interface to directly select the bacterial results.
4.6.4.4 Confirmation of identification results: After the identification states and results are confirmed, you can click the [Confirm to go back] button to exit the current bacterial identification and go back to the identification interface. Then, the bacterial results will be displayed in the corresponding information box synchronously. At the same time, the antibiotic susceptibility category is determined according to transmission of the bacteria category. The [Automatic identification] button and [Manual identification] button in the antibiotic susceptibility identification area are changed from inoperable state to operable state.
4.6.5. Interpretation and analysis of antibiotic susceptibility results
Note: Main form of the analysis system is in an inoperable state when the [Automatic analysis] and [Manual analysis] are carried out.
Note: When interpretation and analysis of the antibiotic susceptibility box is carried out, the KB method can also be used to input the antibiotic susceptibility data.
4.6.5.1 Loading of the kit
Upper cover on interpretation chamber of the identification instrument should be opened, and then the antibiotic susceptibility test kit should be placed into the card slot on interpretation chamber of the instrument.
Note: When placing the kit, please accurately place the kit into the overall large card slot with the chamfering side facing inwards.
4.6.5.2 Automatic analysis: When you click the [Automatic analysis] button in the "antibiotic susceptibility analysis" box after bacterial identification, the instrument will automatically interpret the kit that has been placed and display the detailed test results.
Note: You can click the [Modify results] button to modify state of a certain hole, and then the [All negative] and [All positive] can be restored to operable.
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
4.6.5.3 Manual analysis: You can click the [Manual analysis] in the "antibiotic susceptibility analysis" box to enter the "Manual analysis" operation interface, in which initial state of each hole is negative. When clicking the [MIC Analysis] button after selecting and confirming state of each hole successively, the corresponding antibiotic susceptibility results will be automatically displayed in the list.
Note: You can click the [Modify results] button to modify state of a certain hole, and then the [All negative] and [All positive] can be restored to operable.
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
4.6.5.4 Results modification: If you want to modify state of a hole after antibiotic susceptibility identification by automatic interpretation or manual interpretation, click the [Results modification] button to modify state of one or more holes. When color state of each biochemical item is confirmed after modification, click the [MIC identification] button to get the modified antibiotic susceptibility results.
Note: You can click the [Modify results] button to modify state of a certain hole, and then the [All negative] and [All positive] can be restored to operable.
Note: You can click the [All negative] button to quickly select negative state of all holes; and click the [All positive] to quickly select positive state of all holes.
4.6.5.5 KB method analysis: You can enter the "KB antibiotic susceptibility analysis" by clicking the [K-B analysis] on the "Automatic antibitoic susceptibility identification" or "Manual antibitoic susceptibility identification" interface; you can click the right mouse button and select "Add line", position the mouse in the "Antibiotic name" list box, and then select or enter by double-clicking or pressing the Enter key; and, you can move the mouse to the 4.6.5.1 "K-B" item, press the Enter key first to call out the input box, enter the identification information, and then press the Enter key to confirm your entry operation. You can then move your mouse over the "Sensitivity" tab and finish typing after performing the corresponding operations. After that, you can click "Confirm to go back" to complete the KB method antibitoic susceptibility identification. The KB method antibitoic susceptibility results will be reported simultaneously. After selecting a row, click the right mouse button and select the "Delete row" item in the pop-up menu to complete the operation of deleting the selected row.
4.6.5.6 Confirmation of antibitoic susceptibility results: After confirming the antibitoic susceptibility states and results, click the "Confirm to go back" button to exit the current antibitoic susceptibility analysis and go back to the main operation interface. The antibitoic susceptibility results and expert comments are displayed in the corresponding information box respectively.
4.6.6 Entering of customized result description
4.6.6.1 Click to enter the "Application and identification" interface of the system.
4.6.6.2 Selection of specimens to be tested: Click the item in application list of the specimens to be tested to complete selection of the specimens to be tested.
4.6.6.3 Selection of report date: The report date can be selected by clicking the inverted triangle to the right of the [Report date]. The system defaults to the current system date.
Note: The selected report date cannot be later than the current system date and cannot be earlier than the application date.
Selection of customized result types: Click the "Negative" or "Positive" report selection box under the "Customized results" and select the specific "Negative" or "Positive" report type. Note: When the report type needs to be edited, click the "Basic field settings" tab in the "Settings" menu to enter the "Customized negative results" or "Customized positive results" editing interface to add, modify or delete the report type; or, click the key

followed after the tab to add instantly in case of an emergency.
enter the "Customized negative results" or "Customized positive results" editing interface to add, modify or delete the report type; or, click the key

followed after the tab to add instantly in case of an emergency.
4.6.7 Bacterial identification/antibitoic susceptibility analysis report
4.6.7.1 Saving of bacterial identification/antibitoic susceptibility analysis report: After the operation and confirmation of bacterial identification, antibitoic susceptibility analysis or negative results, click [Save] button to save the information. The saved reports will be displayed in the "Report information" list according to the date, and the information box related to each operation will be restored to the initial state.
4.6.7.2 Printing of report information: Select a valid record in the "Report information" list, click [Print] button to preview the selected report, and then print the selected report.
4.6.7.3 Deletion of report information: When you select a valid record in the "Report Information" list, and then click the [Delete] button, the system will call out the administrator password entry box. After the user enters his/her correct password, the system will give a prompt whether to delete or not. When you select "Yes", deletion will be performed. And when you select "No", deletion will be cancelled.
4.6.7.4 Modification of report information: When you select a valid record in the "Report Information" list, and then click the [Modify] button, the system will call out the administrator password entry box. After entering the correct password, the user can modify the detailed bacterial identification, antibitoic susceptibility analysis or customized reports.
4.6.8 Emptying: You can click the [Empty] button to initialize the antibitoic susceptibility identification.
Notice: Before the identification report is saved, the test description information can be entered by a selected mode or the real-time input mode.
4.6.9 Exit of application and identification
4.6.9.1 To exit the identification application, you can click the "Exit" item in the "Operation" menu, or directly click the button
 in the upper right corner of the operation interface to exit.
in the upper right corner of the operation interface to exit.4.7 Nosocomial infection
4.7.1 You can click the "Nosocomial infection surveillance" in the "Operation" menu to enter the "Nosocomial infection surveillance" interface.
Note: By default, the “Add”, “Modify”, “Delete” and “Print” item in the system are inoperable.
4.7.2 Add: You can select the surveillance date, report date, department name, sampling location, surveillance object, surveillance specimen, surveillance purpose, sampler, tester, surveillance results, microorganism, comprehensive evaluation and test basis successively, and then click the [Add] button to add the current information.
Note: The [Department name], [Sampling location] [Surveillance specimen], [Surveillance object], [Surveillance purpose], [Sampler], [Tester], [Surveillance results], [Comprehensive evaluation], [Microorganism unit], [Test basis] and other items can be entered and set on the setting interface.
Note: The selected report date can be neither later than the current system date nor earlier than the surveillance date.
4.7.3 Modify: When a valid piece of information (that is neither the header nor an empty) in the list is selected, [Add] becomes inoperable, and [Modify], [Delete], and [Print] become operable. After reconfirming the corresponding information, you can click the [Modify] button to modify the current record.
4.7.4 Delete: When a valid piece of information (that is neither the header nor an empty) in the list is selected, [Add] becomes inoperable, and [Modify], [Delete], and [Print] become operable. You can click the [Delete] key to delete the current record.
[Add] becomes inoperable, and [Modify], [Delete], and [Print] become operable. You can click the [Print] key to print the current record.
4.7.5 Initialization: You can click the [Initialization] key to initialize the nosocomial infection surveillance information.
4.7.6 Exit: You can click the [Exit] in the interface
 , or click Note: You can click the "New nosocomial infection surveillance system" in the "Operation" menu to enter the new "Nosocomial infection surveillance system". You can click the "Settings" button in the nosocomial infection surveillance interface to set the basic nosocomial infection fields, or click the "One-key import basic field function" to import the basic fields for settings of the above nosocomial infection surveillance system. Other operations will not be described here.
, or click Note: You can click the "New nosocomial infection surveillance system" in the "Operation" menu to enter the new "Nosocomial infection surveillance system". You can click the "Settings" button in the nosocomial infection surveillance interface to set the basic nosocomial infection fields, or click the "One-key import basic field function" to import the basic fields for settings of the above nosocomial infection surveillance system. Other operations will not be described here.5. Query and statistics
5.1 Query and printing of identification report
5.1.1 You can click the "Query" key on the toolbar, or click the "Microbiological test report query" item in the "Query" menu to query the microbiological test report.5.1.2 Selection of query mode: You can query by the "Report date", "Negative report", "Positive report", "Outpatient/hospitalization number", "Name" and "Specimen number" individually or in any combination. The default mode of the system is to query by the "Report date".
Note: When querying by the "Report Date", the start and end date of the report selected to be queried should not be later than the current system date, and start date of the selected report should not be later than the report deadline date.
Note: When querying by "name", you can enter full name of the test patient for querying, or enter part of his name for fuzzy querying.
5.1.3 Report query: You can select the specific query mode and click the [Query] button. The identification reports that meet the query criteria will be displayed in the current list, and a message will be given if no qualified identification reports are found.
5.1.4 Preview and printing of reports: When a valid record is selected in the list after query, the [Print] button in the query interface will become operable, and you can click this button to preview the selected report. If you want to print the report, click the [Print] button on the preview interface.
5.2 Query of nosocomial infection surveillance reports
5.2.1 You can click the "Nosocomial infection surveillance report query" in the "Query" menu bar to enter the "Nosocomial infection surveillance report query/print" interface.5.2.2 Selection of query mode: You can query the reports by the "Report date", "Department name", "Surveillance object" and "Surveillance specimen" individually or in any combination.
Note: When querying by the "Report Date", the start and end date of the report selected to be queried should not be later than the current system date, and start date of the selected report should not be later than the report deadline date.
5.2.3 Report query: You can select the specific query mode and click the [Query] button. The identification reports that meet the query criteria will be displayed in the current list, and a message will be given if no qualified identification reports are found.
5.2.4 Report printing: When you select a valid record in the list after query, you can click the [Single report print] to print the currently selected report. When you click the [Single report continuous print], you can print one report per page from the currently selected report to the end of the list continuously. When you click the [Multiple report continuous print], you can print four reports per page from the currently selected report to the end of the list continuously. When you click the [List print], you can print interface contents of all reports from the currently selected report to the end of the list. In addition, the [Multiple page print] and data export are also available.
Note: When you want to apply [Single report continuous print], [Multiple report continuous print] and [List print] to all information in the list, you must select the first valid record in the list, because the print in all the three printing modes is started from the currently selected report.
5.3 Bacterial detection rate statistics
5.3.1 You can click the [Bacterial detection rate statistics] in the "Statistics" menu item to enter the "Bacterial detection rate statistics" interface.5.3.2 Selection of statistical date, statistical range and bacterial species: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
Note: When selecting the ward (department), specimen or bacterial species, if you want to select all items, you can click the [Select all] button. If you want to cancel all items currently selected, you can click the [Select none] button.
5.3.3 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.3.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.4 Antibiotic sensitivity statistics
5.4.1 You can click the [Antibiotic sensitivity statistics] in the "Statistics" menu to start the antibiotic sensitivity statistics.5.4.2 Selection of statistical date and bacterial species: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
Note: When selecting the bacterial species, if you want to select all items, you can click the [Select all] button. If you want to cancel all items currently selected, you can click the [Select none] button.
5.4.3 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.4.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.5 Bacteria-antibiotic sensitivity statistics
5.5.1 You can click the [Bacteria-antibiotic sensitivity statistics] in the "Statistics" menu item to enter the "Bacteria-antibiotic sensitivity statistics" interface.5.5.1 Selection of statistical date and bacterial species: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
Note: When selecting the bacterial species, if you want to select all items, you can click the [Select all] button. If you want to cancel all items currently selected, you can click the [Select none] button.
5.5.3 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.5.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface. Statistics of bacterial detection rate in different quarter
5.5.5 You can click the "Statistics of bacterial detection rates in different quarter" item in the "Statistics" menu to start the statistics of bacteria detection rate in different quarter.
5.5.6 Selection of statistical date, statistical range and bacterial species: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
Note: When selecting the ward (department), specimen or bacterial species, if you want to select all items, you can click the [Select all] button. If you want to cancel all items currently selected, you can click the [Select none] button.
5.5.7 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.5.8 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.6 Analysis of bacterial detection trends in different quarter
5.6.1 You can click the [Analysis of bacterial detection trends in different quarter] in the "Statistics" menu item to enter the "Analysis of bacterial detection trends in different quarter" interface.5.6.2 Selection of statistical date, statistical range and bacterial species: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
Note: When selecting the ward (department), specimen or bacterial species, if you want to select all items, you can click the [Select all] button. If you want to cancel all items currently selected, you can click the [Select none] button.
5.6.3 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.6.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.7 Statistics of enzyme-producing strains
5.7.1 You can click the [Statistics of enzyme-producing strains] in the "Statistics" menu item to enter the "Statistics of enzyme-producing strains" interface.5.7.2 Selection of statistical date and statistical purpose: You can click start and end date selection box of the report separately to determine the statistical period, and then click the corresponding statistical conditions separately.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
5.7.3 Statistics: After determining the statistical conditions of the reports to be counted, click the [Statistics] button to conduct statistics.
5.7.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.8 Department submitted quantity statistics
5.8.1 You can click the [Department submitted quantity statistics] in the "Statistics" menu item to enter the "Department submitted quantity statistics" interface.Selection of statistical date: you can determine the statistical period by clicking the report start and end date selection box respectively.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
5.8.2 Statistics: After the report start and end time of to be counted is determined, click the [Statistics] button to conduct statistics.
5.8.3 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.9 Statistics of test specimen count
5.9.1 You can click the [Statistics of test specimen count] in the "Statistics" menu item to enter the "Statistics of test specimen count" interface.5.9.2 Selection of statistical date: you can determine the statistical period by clicking the report start and end date selection box respectively.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
5.9.3 Statistics: After the report start and end time of to be counted is determined, click the [Statistics] button to conduct statistics.
5.9.4 Preview and print of statistical report: After the statistical operation, the preview interface of the statistical report will be displayed. If you want to print the statistical report, click [Print] in the current interface.
5.10 Statistics of tested bacterial count
5.10.1 You can click the [Statistics of tested bacterial count] in the "Statistics" menu item to enter the "Statistics of tested bacterial count" interface.5.10.2 Selection of statistical date: you can determine the statistical period by clicking the report start and end date selection box respectively.
Note: The report start and end date selected for statistics should not be later than the current system date, and the selected report start date should not be later than the report deadline date.
5.10.3 Statistics: After the report start and end time of to be counted is determined, click the [Statistics] button to conduct statistics. The statistical results will be displayed in the current list.
5.10.4 Preview and print of the identification information related to selected bacteria: When you select a valid record in the list and double-click it, the detailed identification information of the selected bacteria will be displayed in its interface list; similarly, once a valid identification report is selected from the list, the report can be previewed and printed.
5.11 Other statistics
In addition, it also includes the statistical functions of "Monthly statistics of specimen count", "Department submission count" and so on.6. System settings
6.1 Purpose of system setting
6.1.1 To facilitate operation, improve the operability and accuracy of the system.6.2 Content items of system settings
6.2.1 The main contents of system settings include: [Mode setting], [Version setting], [Print setting], [Basic field setting], [Nosocomial infection field setting], [Field extension function], [Bacteria threshold setting], [antibitoic susceptibility threshold setting], and [User setting]. Among them, the [Basic field setting] specifically includes the setting items such as "Submitted specimens", "Submission department", "Submission physician", "Test purpose", "Clinical diagnosis", "Customized negative result", "Customized positive result", "Customized comments", "Test description" and "Reviewer".6.3 Setting operation of the system
Settings of [Mode setting]6.3.1.1 You can click the [Mode setting] item in the "Settings" menu to enter the "Mode setting" interface The [Mode setting] includes the report LIS setting, drug resistance mechanism mode, user type and other settings. Note: When selecting the [Quality control mode], the antibitoic susceptibility kit of corresponding version should be used together, and the corresponding antibitoic susceptibility interpretation version should be set in the [Version setting].
6.3.1 Settings of [Mode setting]
6.3.1.2 You can click the [Version setting] item in the "Settings" menu to enter the "Version setting" setting interface. The [Version setting] includes the bacteria category selection, kit settings and antibitoic susceptibility report settings.
6.3.2.1 In the [Version setting] interface, the default bacteria category is enterobacter. You can select and set as staphylococcus, non-fermentative bacteria and streptococcus respectively from the drop-down menu, and make relevant settings.
Note: The kit setting here should be set according to the type of kit used by the user. If the setting here do not match with the actual kit used, the report will be incorrect. For the type of kit, please refer to the kit packaging label or consult the appropriate personnel.
Note: The "Antimicrobial drugs in report CLSI standard" is generally selected in the "antibitoic susceptibility report setting". If the "All antimicrobial drugs on the report antibitoic susceptibility plate" is selected, the antibitoic susceptibility results will not be shown and will be represented by “N/R” because some drugs do not have antibitoic susceptibility beak-point.
6.3.3 Settings of [Print setting]
6.3..3.1 You can click the [Print setting] item in the "Settings" menu to enter the "Report print setting" setting interface. The [Print setting] includes selection of paper settings and selection of print report content settings.
6.3.4 Settings of [Basic field setting]
6.3.4.1 You can click the [Basic field setting] item in the "Settings" menu to enter the "Basic field setting" setting interface. The [Basic field setting] includes the setting items such as "Submitted specimens", "Submission department", "Submission physician", "Test purpose", "Clinical diagnosis", "Customized negative result", "Customized positive result", "Customized comments", "Test description" and "Reviewer". The settings of "Submitted specimens" will be taken as an example in the following paragraphs to describe the operations of "Add", "Modify", "Delete" and "Delete all".
6.3.4.2 Add: You can select the "Submitted specimens" item, enter corresponding contents in the blank box under "Operation" - "Submitted specimens" on the right side of the interface, and then click the "Add" button below to add the specimen. The added specimen will be displayed in the middle of the interface.
6.3.4.4 Modify: You can select the specimen to be modified under the "Select content" in the middle of the interface, enter corresponding contents in the field of "Submitted specimens" under "Operation" on the right, and click the "Modify" button below to modify corresponding specimen.
6.3.4.4 Delete: You can select the specimen to be deleted under the "Select content" in the middle of the interface, and click the "Delete" button at the lower right to delete corresponding specimen. If you want to delete all submitted specimens, you can directly click the "Delete All" button on the right side of the interface to delete.
The settings of other basic fields, including the [Nosocomial infection field setting], are similar and will not be described in detail here.
Note: The settings of the basic fields are mainly for the convenience of "Specimen addition", "Bacterial identification" and other operations. Users can set it here in advance, or add it temporarily (as described above) during the operation of "Specimen addition", "Bacteria identification", etc. After addition, it will be automatically saved here.
6.3.5 Settings of [User setting]: This setting includes the functions such as "User login information management", "User management" and "Password modification".
6.3.5.1 [User login information management]: You can click the "User login information management" item in the "User" menu to enter its operation interface.
Information query: The login information can be queried by name and time. Specifically, when
you select the time or name and click the [Query] key, the information that meets the criteria will be displayed in the corresponding list.
6.3.5.1.1 Delete/delete all: You can select certain information in the list and click [Delete] to delete the current information; and, you can click the [Delete all] to delete all the information in the list.
6.3.5.1.2 Exit: You can click the [Exit] button in the interface
 or click
or click6.3.5.2 [User management]: You can click the "User management" item in the "User" menu to enter its operation interface.
6.3.5.2.1 When you want to add users, you can click the [Add] button to enter the "User management - Add" interface. After confirming the entered and selected items, you can click the [Save] button to complete addition of users.
Note: The [Select all] and [Select none] are the keys that can be used to quickly select or cancel permission settings.
Note: The [Cancel] key is used to initialize the user information before saving it.
Note: After saving the current information, the information of each input box will be initialized, and the user information can be entered in batches.
Note: At the end of the current operation, you can press the [Back] key to exit the current operation and go back to the user management interface.
6.3.5.2.2 When you want to modify the user information, select the specified valid information in the list, and then click [Modify] key to enter the "User management - Modify" operation interface.
Note: The [Select all] and [Select none] are the keys that can be used to quickly select or cancel permission settings.
Note: The [Initialize] key is used to initialize the user information before it is saved.
Note: At the end of the current operation, you can press the [Back] key to exit the current operation and go back to the user management interface.
6.3.5.2.3 Delete: When you want to delete the user information, click the [Delete] button to delete the selected item.
6.3.5.2.4 Exit: You can click the [Exit] key or the
 at upper right corner of the "User management" interface to exit the current interface.
at upper right corner of the "User management" interface to exit the current interface.6.3.5.3 [Password modification]: You can click the "Password modification" item in the "User" menu to enter its operation interface.
6.3.5.3.1 You can enter the user's current password, enter the new password and confirmation password, and then click [OK] to modify the password.
Note: The new password entered and the confirmation password should be the same.
Note: The user "xk" is the system user and its password cannot be modified.
6.3.5.4 After the [Login] is cancelled, the system will be locked. When the user wants to do the operation again, he/she should click the
 button in the toolbar or click the "Login" item in the "User" menu to log in.
button in the toolbar or click the "Login" item in the "User" menu to log in.6.3.5.5 The [Cancel] is to ensure security of the system. When exiting current operation of the system, the user should click the
 button in the toolbar or click the "Logout" item in the "User" menu to lock the system.
button in the toolbar or click the "Logout" item in the "User" menu to lock the system.Note: After logging out, the system needs to be logged in again before it can be operated again.
Note: The other settings, such as "Field extension function", "Bacterial threshold setting" and "antibitoic susceptibility threshold setting", are not allowed to be set by users themselves.
7. Tools
7.1 Antibiotic data
7.1.1 When the user wants to view the antibitoic susceptibility identification parameters, click the "Antibiotic data" item in the "Tools" menu to enter the "Antibiotic data" interface.7.2 antibitoic susceptibility test description
7.2.1 StartYou can click the “antibitoic susceptibility test description” key in the toolbar or the “antibitoic susceptibility test description” item in the “Tools” menu to start the “antibitoic susceptibility test description”
7.2.2 Application
In the “antibitoic susceptibility test description”, you can find information on the basic procedures for antibitoic susceptibility testing in the latest CLSI standard.
7.2.3 Exit
You can click the
 key in the upper right corner to exit the current operation.
key in the upper right corner to exit the current operation.7.3 LIS comparison table
7.3.1 Start: You can click the "LIS comparison table" item in the "Tools" menu to enter the "LIS comparison table" interface.7.4 WHONET
7.4.1 Start: You can click the "WHONET data export" under "Tools" to start the data export operation. You can determine the data export conditions and data, click the "Data export" key to export the data.7.4.2 Exit: You can click the "Exit" key, or click the
 key in the upper right corner to exit the current operation.
key in the upper right corner to exit the current operation.7.5 Backup/restore
7.5.1 You can click the "Backup/restore" item in the "System" menu to enter the "Backup/restore" interface.7.5.2 Backup: You can click the
 icon in the interface to set the backup path, and then click the [Backup] button to complete the backup operation.
icon in the interface to set the backup path, and then click the [Backup] button to complete the backup operation.7.5.3 Restore: If the backup data needs to be restored, you should select the backup file path, select the data to be restored, and then click the [Restore] button to restore.
8. Help
8.1 About
8.1.1 StartYou can click the “About” item in the “Help” menu to start the “About” operation.
8.1.2 Application
With the “About”, you can view software version and other information about the identification instrument.
8.1.3 Exit
You can click the
 key in the upper right corner to exit the current operation.
key in the upper right corner to exit the current operation.9. Use and maintenance of the instrument
9.1 Working conditions
- For indoor use;- Ambient temperature: 5℃ - 40℃;
- Relative humidity: ≤70%;
- Power supply: AC220V±10%, 50Hz±10%;
- Input power: 400VA;
- The interference source of strong electromagnetic field should be far away, and the direct irradiation of strong light should be avoided;
- The working site shall be protected from dust and vibration; the air shall not contain any acid, salt and other corrosive gases;
- The grounding environment shall be good;
- The space with the wall should be no less than 20cm, in order to protect the power supply and data cable, and to facilitate the heat dissipation;
- There shall be sufficient operation and maintenance space for the operators, and requirements of the relevant regulations shall be met.
- The instrument shall not be placed in a position that will prevent the operator from switching on and off power supply of the instrument normally;
- It needs to be warmed up for 10 minutes.
9.2 Scope of application
It is applicable for the qualitative identification of common clinical pathogenic bacteria (enterobacteriaceae, non-fermentative bacteria, staphylococcus, streptococcus, yeast-like fungi) and semi-quantitative analysis of antimicrobial susceptibility.9.3 Contraindications
None9.4 Cautions, warnings and suggestive instructions
9.4.1 It is recommended to be used in a Level 2 biosafety laboratory, and attention should be paid to biosafety protection during operation;9.4.2 The medical wastes generated in the test shall be treated harmlessly according to the requirements of the local industry;
9.4.3 Input and output instructions
9.4.3.1 The USB port of the device can be used to connect the keyboard, mouse, printer, and to copy data to the outside. It is prohibited to use it for other purposes other than the above data exchange, especially to supply power to peripheral equipment;
9.4.3.2 The devices with a network interface should be avoided from connecting to the external network as far as possible;
9.4.3.3 If the power cord is plugged and unplugged from power source of the equipment, the power should be cut off before operation to prevent electric shock;
9.4.4 The working table should be flat and have enough space;
9.4.5 It should be kept away from the heat source. The normal working environment temperature is 20℃ at room temperature and should not exceed 40℃;
9.4.6 Excessive humidity and dust in the working environment shall be avoided;
9.4.7 Sharing the same power supply with high-power or interference-generating electrical devices such as refrigerators should be avoided, in order to keep the power supply stable;
9.4.8 The power supply voltage shall be AC220V±10%, 50Hz±10%;
9.4.9 The mainframe and supporting devices should be installed reasonably;
Surface of the instrument and the kit interpretation tray should be cleaned after the power is turned off on a regular basis.
9.4.10 Attention shall be paid to the loss, damage and loosening of the instrument parts and screws frequently, and timely treatment and repair shall be made if the above problems are found;
9.4.11 Foreign objects, liquid and corrosive substances shall be prevented from entering the instrument interface;
9.4.12 It is strictly prohibited to plug and unplug the on-line cable of the instrument when it is live, so as not to damage the instrument interface;
9.4.13 Attention should be paid to the amount, thickness and excessive dampness of printer papers frequently;
9.4.14 The instrument shall be operated in strict accordance with the operation procedures. All switches shall be turned on or off gently, and shall not be hit by hard objects;
9.4.15 The instrument is an in vitro diagnostic medical device (IVD);
9.4.16
 The test specimens of the instrument are potentially infectious, so the "Biological hazard" label is posted at an obvious location of the instrument:
The test specimens of the instrument are potentially infectious, so the "Biological hazard" label is posted at an obvious location of the instrument:9.4.17 When the instrument is powered on, the power supply parts shall not be touched to prevent danger. The “Be careful, dangerous" mark shall be affixed to the corresponding part:
9.4.18
 If the instrument is not used in accordance with the method described in the Manual, the instrument may fail to operate normally and/or output error data information and all the consequences arising shall be borne by the using unit and/or user.
If the instrument is not used in accordance with the method described in the Manual, the instrument may fail to operate normally and/or output error data information and all the consequences arising shall be borne by the using unit and/or user.9.4.19 The dust on external surface of the instrument and the reagent plate tray should be cleaned regularly;
9.5 Maintenance methods
9.5.1 Precautions before maintenanceMaintenance of the instrument must be carried out under the most appropriate conditions.
9.5.1.1 The operator shall wear special work clothes and gloves before operation.
9.5.1.2 All internal power must be disconnected before maintenance of the instrument.
9.5.1.3 Thinners, trichloroethylene or ketones should not be used to wipe all plastic components.
9.5.1.4 All switches should be turned on and off gently.
9.5.2 Preparations for cleaning of interpretoscope
9.5.2.1 Check that all cables, wires and plugs are safe.
9.5.2.2 Check whether all plate holes are clean.
9.5.3 Periodic maintenance of interpretoscope
9.5.3.1 Check whether any external screw of the instrument is loose every six months;
9.5.3.2 Weekly cleaning of identification meter surface and kit carrier: gently wipe with a piece of clean, damp and dripless cloth.
The 75% alcohol should be used for cleaning and disinfection every week to avoid biological
infection.
9.5.4 Rating and characteristics of fuses:
There are 2 F2AL250V fuses with the characteristics of current-limiting protection in the instrument;
9.5.5 Parameters and disposal methods of battery
9.5.5.1 The keyboard and mouse are powered by triple-A battery, and voltage of each battery is 1.5V. The waste batteries should be effectively disposed of according to local environmental protection policies, regulations and management measures, and must not be discarded at will.
9.5.5.2 When replacing the battery, remove the battery cover according to the arrow on the corresponding battery. When installing the battery, attention should be paid to the direction as the side marked with "+" is positive, and the side marked with "-" is negative.
9.6 Warranty period
9.6.1 The warranty period of this product is one year from the date of purchase, and the designed service life is ten years. For other details, please refer to the warranty card.9.7 Discontinuation of the equipment due to maintenance, transportation or handling
- Before implementing this step, please read this Manual carefully and follow the instructions.- If the whole equipment needs to be thoroughly cleaned and disinfected, the power should be turned off in time. The operator should wear special work clothes and gloves before operation.
- If any maintenance is necessary in case of equipment failure, please read this Manual carefully, power off the equipment first, and clean and disinfect the areas that may be touched by human body with 75% alcohol to avoid biological infection;
- If the equipment needs to be moved or returned to the factory for maintenance, the equipment should be cleaned and disinfected according to the requirements of this Manual.
- The equipment is a precision testing instrument, so care should be taken during transportation and storage to avoid it from being damaged. The words and marks such as "Handle with care", "Up" and "Keep dry" should be marked on the outer packing box. If the distance is long, the transportation department or unit must protect against severe shock, rain and sunlight exposure during the transportation to the designated point.
9.8 Common faults and troubleshooting
| Types | Possible causes | Solutions |
| The monitor does not work | Signal line of the monitor is not connected firmly, or the mainframe is not turned on properly | First check whether signal line of the monitor is firmly connected with the mainframe interface, and then check whether the mainframe is normally turned on; If the mainframe cannot be turned on normally, please contact authorized engineer of the manufacturer |
| The USB device cannot be identified or the USB device is not stable | The USB interface is loose | Reconnect the USB device, or contact authorized engineer of the manufacturer |
| The interpretoscope tray is running idle | The boot sequence is incorrect | Please turn on the interpretoscope switch again |
| The interpretoscope tray is stuck | Other causes | Turn off the interpretoscope switch, and contact authorized engineer of the manufacturer |
| The interpretation system does not respond | The fuse is burnt out or other faults | Please disconnect power supply of the interpretation system and check whether the two fuses are normal. If they are burned out, please replace the fuses with the ones of the same type. Otherwise, please contact authorized engineer of the manufacturer |
| The printer does not work properly | The printer driver is not installed properly, or the print setup is not normal | Reinstall the printer driver; or carry out the print setup again |
| The software cannot be run, or runs slowly | Connection of an USB device cause viruses in instrument software | Please contact authorized engineer of the manufacturer |
| Kit hole state interpretation result is abnormal | The kit to be interpreted is not placed, or other faults | Check if any kit to be interpreted is placed. Otherwise, contact authorized engineer of the manufacturer |
Note: This product is a precision instrument, in which the whole machine includes the circuit box, precision machinery and precision mechanisms of supporting products. Therefore, care must be taken during repair and maintenance, and the "Cautions, warnings and suggestive instructions" shall be read before starting.
Note: This product is made of precision components and advanced technologies, which are exclusively produced by our company. Therefore, any machine damage caused by replacement of components with the ones not produced by our company, disassembly by customers themselves, improper operation or unexpected reasons, which affects the use effect or even causes the machine to be scrapped, the responsibility shall be borne by customers themselves.
10. Electromagnetic compatibility declaration
1. Users shall ensure the electromagnetic compatibility environment of the device to enable the device to work normally. It is recommended to assess the electromagnetic environment prior to use of the device;2. Basic performance
-Absolute value of the measurement accuracy of the BCMD-101 should be ≤0.15.
-When it is working, both the monitor and the indicator lights shall work normally.
-It can print normally.
3. Test methods

- The wavelength of 492nm should be selected, air should be used as a reference, and then the calibrated standard glass plate with a nominal value of about 1.5A should be placed in specimen position of the instrument kit tray for continuous measurement for 3 times. The test results of any selected hole shall be calculated according to formula (1), and the absolute value of measurement accuracy shall be ≤0.15.
Wherein: Ai -- absorbance value measured at the ith time; As -- calibrated value of absorbance.
- During visual inspection, the monitor should work normally, and states of the indicator lights should correspond to the working modes one by one.
- After generating the report, execute the printing operation to print.
10.1 Sample cables
Table 11-1 Sample cables (m)| Specification model | XK | XK-Ⅱ |
| BCMD-101 mainframe power cord | 1.5 | 1.5 |
| Computer mainframe power cord | 1.5 | - |
| Computer monitor VGA video cable | 2 | - |
| Computer monitor power cord | 1.5 | - |
| Keyboard cable | 1.6 | - |
| Mouse cable | 1.6 | - |
| Printer power cord | 1.5 | - |
| Printer data cable | 2 | - |
Warnings:
1) This device should not be used in proximity to other devices or stacked with other devices. If this is unavoidable, it should be observed and verified that it works properly in its usage configuration.
2) Use of other electrical devices on or near this product may cause interference. Before using the device for identification, check that the device is working as you configured it.
3) It is forbidden to use the device near strong radiation sources (e.g. unshielded RF sources), otherwise it may interfere with normal operation of the device.



